0885
Pre-surgical structural and functional MRI to optimize cerebellar stimulation electrode placement for chronic stroke therapy1Cleveland Clinic, Cleveland, OH, United States, 2Santa Casa de Belo Horizonte Hospital, Belo Horizonte, Brazil, 3Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, 4Universidade Federal de Sao Paulo, Sao Paulo, Brazil
Synopsis
Keywords: Stroke, fMRI (resting state), deep brain stimulation
To improve reproducibility of positive outcomes from cerebellar dentate stimulation for chronic stroke, we hypothesized that the volume of tissue activated (VTA) (dentateVTA) proximity to the volume functionally connected to ipsilesional motor-associated cortices (dentateFC-ipsi-motor) would be associated with improved arm impairment/function metrics. DentateVTA was estimated using computed tomography and T1-weighted MRI. DentateFC-ipsi-motor was estimated from fMRI (resting state). Significant correlations with arm metric improvement were not found for dentateVTA proximity to dentateFC-ipsi-motor, but were found for dentateVTA size, distance from center, and percentage VTA in the inferior-lateral-posterior region. These findings suggest stimulating a small target at the inferior-posterior-lateral dentate edge.Introduction
Stroke can cause crossed cerebellar diaschisis, characterized by reduced metabolism within the contralesional cerebellum. In a phase I clinical trial, stimulation of the contralesional cerebellar dentate nucleus enhanced the effects of rehabilitation of hand motor deficits in the majority of chronic post-stroke participants.In the clinical trial, the electrode was placed based on neurosurgical experience. We hypothesized that simulation close to the dentate region with residual functional connectivity to the ipsilesional motor-associated cortices (dentateFC-ipsi-motor) could improve the reproducibility of positive outcomes. As the first step to testing this hypothesis, we measured the association between proximity of the dentate volume of tissue activated (VTA) by electrode stimulation (dentateVTA) to dentateFC-ipsi-motor and change in arm impairment and function metrics. We also measured the independent associations between dentateFC-ipsi-motor and dentateVTA and change in arm metrics. Finally, we measured the association between the VTA location within the dentate and change in arm metrics.
Methods
Data was available in 8 chronic stroke participants.Clinical metrics: Upper-extremity impairment was quantified using the Fugl-Meyer Assessment (FM-UE), and arm function was quantified using the Arm Motor Ability Test (AMAT). The change in arm metrics ΔAMAT and ΔUEFM were calculated as the difference in score immediately after stimulation was stopped relative to prior to the start of stimulation.
MRI acquisition: T1-weighted (T1w) MRI was acquired at 3 tesla (T), using magnetization prepared rapid gradient echo imaging with 192 1mm-thick slices, in-plane resolution=1x1mm2. Resting-state functional MRI (rs-fMRI) were acquired at 7T in awake patients with eyes closed, using a simultaneous multi-slice echo-planar-imaging with 81 contiguous 1.5mm-thick axial slices, in-plane resolution=1.2x1.2mm2, 128 volumes.
VTA estimation: For comparisons of VTA and functional connectivity, VTA estimation was performed using the Lead-DBS toolbox1 with the SimBio/Fieldtrip pipeline2. Atlas-based3 segmentation used the pre-implant T1w MRI, and lead localization used the co-registered postoperative computed tomography imaging. Modeling parameters matched participants’ clinical settings. For measuring the association between the VTA location within the dentate and change in arm metrics, data from VTAs previously quantified using Guide XT (Boston Scientific) were used.
Dentate metrics:
· The dentate was manually segmented on the average rs-fMRI volume. The ipsilesional Human Motor Associated Template (HMAT4) was warped to rs-fMRI-space. For each dentate voxel, the associated z-map was calculated and significant clusters5 were identified. The dentate voxel was classified as dentateFC-ipsi-motor if the associated z-map contained a significant cluster with a center within the ipsilesional HMAT. To minimize the effects of noise in the z-maps on voxel classification, z-maps with widespread significant clusters within and outside the ipsilesional HMAT on both ipsilesional and contralesional sides were omitted.
· The dentate VTA was warped to rs-fMRI-space to calculate overlapFC-VTA=(dentateFC-ipsi-motor ∩ dentateVTA)/|dentateFC-ipsi-motor|. To overcome noise in the voxel classification, we also measured distanceFC-VTA, the distance between the centers of dentateFC-ipsi-motor and dentateVTA.
· To characterize dentateFC-ipsi-motor and dentateVTA independently, we calculated their sizes relative to the dentate size (sizeFC/Den and sizeVTA/Den) and the distance between their centers from the dentate center (distanceFC-Den and distanceVTA-Den).
· Using Matlab (2020b, The MathWorks, Inc.), a principal components analysis of the dentate was performed to quantify the percentage of the VTA within 8 dentate regions: superior-anterior-medial, superior-anterior-lateral, superior-posterior-medial, superior-posterior-lateral, inferior-anterior-medial, inferior-anterior-lateral, inferior-posterior-medial, inferior-posterior-lateral.
Statistical analyses: Statistical analyses were performed using Matlab. Pearson and Spearman correlations were used to evaluate the associations between MRI metrics and change in arm function metrics.
Results
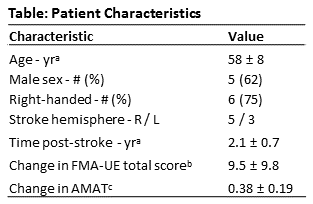
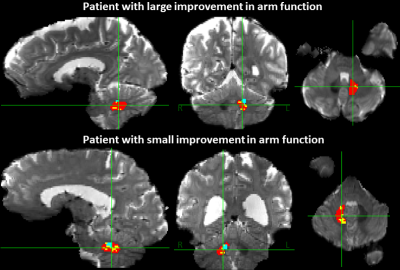
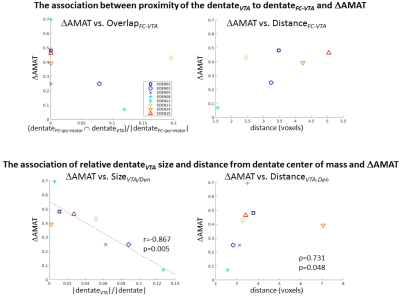
The patient demographics, stroke characteristics and change in arm metrics are shown in the Table. Figure 1 shows the contralesional dentate, VTA, and dentateFC-ipsi-motor segmentations for a participant who experienced a large improvement of arm function and a participant who experienced a small improvement of arm function. There were no statistically significant correlations between overlapFC-VTA and distanceFC-VTA and ΔUEFM or ΔAMAT (Fig. 2, top). Statistically significant correlations were found between sizeVTA/Den and ΔAMAT (r=-0.867, p=0.005, Fig. 2, bottom-left) and between distanceVTA-Den and ΔAMAT (ρ=0.731, p=0.048, Fig. 2, bottom-right), but not with ΔUEFM. Correlations between sizeFC/Den and distanceFC/Den and ΔUEFM or ΔAMAT were not significant. The percentage of VTA in the inferior-posterior-lateral dentate significantly correlated with ΔUEFM (r=0.879, p=0.004), but not for the other dentate regions or with ΔAMAT.Discussion
We hypothesized that dentateVTA proximity to dentateFC-ipsi-motor would be associated with improved arm metrics. This was not supported by linear correlation, however statistically significant correlations between sizeVTA/Den and distanceVTA-Den and ΔAMAT suggested that ΔAMAT was associated with a smaller-sized VTA located away from the dentate center. To improve VTA characterization, additional correlation analyses between VTA location and change in arm metrics revealed a significant correlation between the percentage of VTA in the inferior-posterior-lateral dentate and ΔUEFM. Together the findings suggest that a small-sized VTA located at the inferior-posterior-lateral dentate edge may provide positive therapeutic outcomes. These findings are limited by several factors, including: underestimation of dentateFC-ipsi-motor due to noise, inaccuracies in VTA estimation, and inaccuracies in measuring overlap and distances due to inaccuracies in image registration. Future studies will quantify how the dentateFC-ipsi-motor changes with stimulation.Conclusion
These findings suggest that electrode placement in the contralesional dentate for chronic stroke therapy should avoid stimulating the dentate widely, and target a small region at the inferior-posterior-lateral dentate edge.Acknowledgements
This work was supported by the National Institutes of Health - [UH3-NS100543] and Enspire DBS Therapy, Inc.References
1. Horn A and Kühn AA: Lead-DBS: A toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage; 2015;107:127-135.
2. Horn A, Reich M, Vorwerk J, et al. Connectivity Predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol. 2017;82(1):67-78.
3. Diedrichsen J, Maderwald S, Küper M, et al. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. Neuroimage; 2011;54(3):1786-1794.
4. Mayka MA, Corcos DM, Leurgans SE, et al. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage; 2006; 31(4):1453-1474.
5. Jenkinson M, Beckmann CF, Behrens TEJ, et al. FSL. Neuronimage; 2012; 62(2):782-790.
Figures