0867
Navigator-free water/fat separation for multi-shot diffusion-weighted EPI using structured low-rank reconstruction1C.J. Gorter MRI Center, Department of Radiology, LUMC, Leiden, Netherlands, 2Philips, Best, Netherlands, 3Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom, 4Philips Research, Hamburg, Germany
Synopsis
Keywords: Image Reconstruction, Diffusion/other diffusion imaging techniques
Multi-shot EPI readout-approaches provide high spatial resolution at reduced geometric distortions and improved SNR in diffusion weighted imaging (DWI). As a specific challenge, physiological motion induces shot-to-shot phase variations and needs specific handling, e.g., using additionally measured phase navigators, data-driven phase estimation and/or low-rank regularizations. Furthermore, good fat-suppression is also needed in DWI, making the use of chemical-shift encoding interesting. In this work, a structured low-rank-based water/fat separation pipeline is proposed to jointly estimate water/fat images while correcting motion-induced phase variations with improved time efficiency. In-vivo examples from different anatomies demonstrate improved water/fat separation compared to conventional approaches.Introduction
In EPI-based diffusion-weighted imaging (DWI), fat is always a confounding factor, especially in regions of large B0 inhomogeneities, where conventional fat-saturation is prone to failure. To address this challenge, chemical-shift encoding (Dixon) has been combined with DWI1,2. Furthermore, multi-shot EPI has gained popularity in DWI to increase image resolution and reduce geometric distortions3. But at the same time, extra-navigation3, or self-navigation methods4 become necessary to cope with physiological motion-induced shot-to-shot phase variance. However, when combining multi-shot EPI DWI and Dixon, the fat signals present in the extra-navigators are not easily removed and may thus cause problems. Self-navigation/navigation-free methods can potentially solve this with reduced scan time. Inspired by MUSSELS5, we propose an iterative, model-based reconstruction pipeline with two individual structured low-rank regularizations acting respectively on water/fat channels to deal with the shot-to-shot phase variations while separating water/fat images. In-vivo experiments in different anatomies demonstrate its effectiveness compared to extra-navigated/SPIR results.Methods
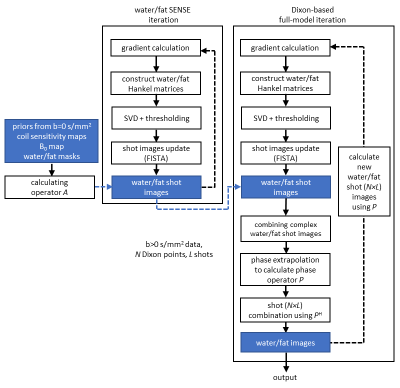
The cost function of the chemical-shift encoded multi-shot DWI-EPI can be written as:$$\left\{\bar{x}_w,\bar{x}_f\right\}=\underset{x_w,x_f\in\mathbb{R}^Q}{\operatorname{argmin}}\|A{P}{x}-d\|_2^2+\lambda_1\left\|H\left(P_w{x_w}\right)\right\|_*+\lambda_2\left\|H\left(P_f{x_f}\right)\right\|_*,\qquad\qquad{(1)}$$where $$$Q$$$ is the number of voxels, $$$P$$$ contains $$$P_w$$$ and $$$P_f$$$ on its diagonal blocks adding the diffusion phase for individual water/fat shots, $$$x=\left[x_w,x_f\right]^T$$$ the joint water/fat magnitude images, $$$d$$$ the vectorized k-space data for N Dixon points, L shots, and J coils, $$$\lambda_1/\lambda_2$$$ the regularization factors for water/fat channels. $$$\left\|H\left(P_{w/f}x_{w/f}\right)\right\|_*$$$ are the block-Hankel regularization terms similar as introduced in the MUSSELS5 which enforce the low-rankness between different shots of the water/fat channels, separately, through the nuclear norm. The low-rank constraint leverages the redundancy across shots by assuming that their underlying magnitude components are equal despite their different phases. It should be noted that water/fat signals are assumed to have one joint phase, after correcting for the spatial displacement of fat for each shot. This joint, but shot-specific phase, is described by the phase operator $$$P$$$ (shape $$$2\times{N}\times{L}\times{Q},2\times{Q}$$$), which also introduces the Dixon dimension into the cost function. The matrix $$$A$$$ can be expressed as:$$A=K\left[\begin{array}{ll}I&I\end{array}\right]\left[\begin{array}{cc}F{S}{\Psi_B}&0\\0&\Psi_f{F}S\Psi_B\end{array}\right],\qquad\qquad{(2)}$$where $$$K$$$ indicates the shot-specific k-space undersampling, $$$I$$$ is the identity matrix, $$$\Psi_f$$$ adds fat off-resonance, $$$S$$$ adds the coil sensitivity weighting, $$$\Psi_B$$$ adds the B0 inhomogeneity-induced phase, which are implemented through linear operators. For the DWI reconstruction, the B0 map prior (and two thresholding water/fat masks) and coil-sensitivity maps can be calculated/calibrated using an image-based water/fat decomposition approach for EPI (IDE)2 and ESPIRiT7 as described in ref.6 from the b = 0 s/mm2 data. For all reconstructions, a multi-peak fat model was used. The general reconstruction pipeline is shown in Figure 1. The water/fat images were initialized by first minimizing:$$\left\{\bar{m}_w,\bar{m}_f\right\}=\underset{m_w,m_f\in\mathbb{C}^{N\times{L}\times{Q}}}{\operatorname{argmin}}\|A{m}-d\|_2^2+\lambda_3\left\|H\left(m_w\right)\right\|_*+\lambda_4\left\|H\left(m_f\right)\right\|_*,\qquad\qquad{(3)}$$where $$${m}=\left[m_w,m_f\right]^T$$$ is the collection of all complex water/fat shot images (L$$$\times$$$N). In this case, since the operator $$$P$$$ is dropped in Eq.3, the water/fat separation for each l and n can be treated as a SENSE-based separation scenario (water-fat SENSE6-9), benefiting from the chemical-shift-induced spatial displacement of fat, using SENSE to disentangle water and fat. In Eq.3, this step is also guided by structured low-rank regularization.Experiments were conducted in healthy volunteers (after informed consent was obtained) in brain, knee and head-neck regions at 3T (Philips, Best, The Netherlands), using chemical-shift encoded spin-echo DW four-shot-EPI2,6, with b-values of 0,1000s/mm2 for brain and 0,300,600s/mm2 for other anatomies. A 16-channel head-neck and 8-channel knee coil were used, resolution: 1×1×4mm³ (brain), 1.4×1.5×4 mm3 (head-neck/knee) with TE=120ms/TR=5000ms or TE=70/TR=5000ms (no partial-Fourier). For water/fat encoding, three echoes of $$$\Delta{TE}$$$ were chosen as 0.2/1.0/1.8ms with respect to the spin echo, enabled by shifting the msh-EPI sampling window back and forth. An extra-navigator was acquired for each diffusion shot for comparison2,6. One scan in the head-neck was repeated with conventional fat suppression (SPIR10). Moreover, fully sampled data were retrospectively undersampled. All hyper parameter tuning and the implementation of Hankel-matrices were done as in ref.11 with filter size of 8×8. Eq. 1 and Eq. 3 were solved using 20 iterations of each.
Results
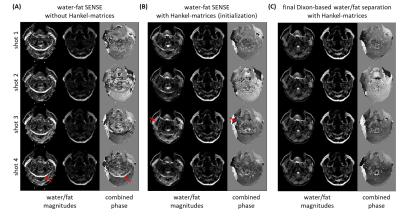
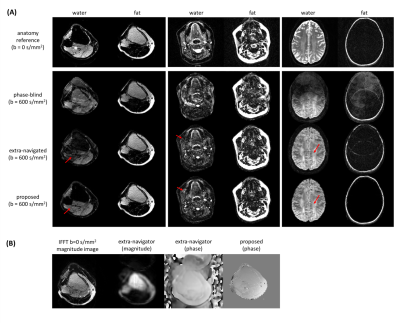
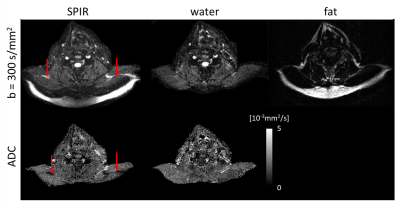
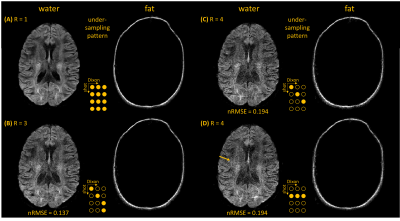
Figure 2 shows water/fat, magnitude/phase shot-images of one Dixon point, comparing water-fat SENSE separation without (A) and with (B) low-rank-regularization, and (C) the structured low-rank-regularized full-model Dixon-based water/fat separation. Figure 3 shows water/fat images comparing reconstruction without navigation (phase-blind), with extra-navigators, and with the proposed structured low-rank full-model reconstruction. An example navigator image of the leg slice, which has shifted fat present, was also shown to compare with the fat-displacement-corrected phase map from the proposed method. Figure 4 shows a comparison between water/fat images from SPIR and from the proposed approach in a B0 critical region, with corresponding ADC maps. Figure 5 shows reconstruction results of the proposed approach using different k-space undersampling patterns for the Dixon/multi-shot dimensions.Discussion and conclusion
In this work, we used low-rank regularizations to enable navigator-free water/fat separation for multi-shot diffusion-weighted EPI. The results showed that this approach produces superior image quality with shorter scan times compared to both extra-navigation and SPIR techniques. Although Dixon is a smart way of signal averaging, it is also demanding for robust water/fat separation and scanning time. However, in this work, we showed the ability of the proposed approach to support k-space undersampling, when covering the full k-/Dixon-space extent. Thus, no scan time penalty has to be paid, allowing to get Dixon-based water/fat separation in ms-DWI-EPI for “free”.Acknowledgements
The authors would like to acknowledge NWO-TTW (HTSM-17104).References
1. Burakiewicz J, Charles-Edwards DG, Goh V, Schaeffter T. Water-fat separation in diffusion-weighted EPI using an IDEAL approach with image navigator. Magn Reson Med. 2015 Mar;73(3):964-72.
2. Dong Y, Koolstra K, Riedel M, van Osch MJP, Börnert P. Regularized joint water–fat separation with B0 map estimation in image space for 2D-navigated interleaved EPI based diffusion MRI. Magn Reson Med. 2021; 00: 1– 18.
3. Jeong H-K, Gore JC, Anderson AW. High-resolution human diffusion tensor imaging using 2-D navigated multishot SENSE EPI at 7 T. MRM. 2013;69(3):793-802.
4. Butts, K., Pauly, J., De Crespigny, A. and Moseley, M. (1997), Isotropic diffusion-weighted and spiral-navigated interleaved EPI for routine imaging of acute stroke. Magn Reson Med., 38: 741-749.
5. Mani M, Jacob M, Kelley D, Magnotta V. Multi-shot sensitivity-encoded diffusion data recovery using structured low-rank matrix completion (MUSSELS). Magn Reson Med. 2017;78(2):494-507.
6. Dong, Y, Riedel, M, Koolstra, K, van Osch, MJP, Börnert, P. Water/fat separation for self-navigated diffusion-weighted multishot echo-planar imaging. NMR in Biomedicine. 2022;e4822. doi:10.1002/nbm.4822
7. Uecker M, Lai P, Murphy MJ, et al. ESPIRiT - An eigenvalue approach to autocalibrating parallel MRI: Where SENSE meets GRAPPA. Magn Reson Med. 2014;71(3):990-1001.
8. Uecker M. Making SENSE of Chemical Shift: Separating Species in Single-Shot EPI using Multiple Coils. ISMRM. 2012;20:2490.
9. Shin PJ, Larson PEZ, Uecker M, et al. Chemical shift separation with controlled aliasing for hyperpolarized 13C metabolic imaging. Magn Reson Med. 2015;74(4):978-989.
10. Zee CS, Segall HD, Terk MR, et al. SPIR MRI in spinal diseases. J Comput Assist Tomogr. 1992;16(3):356-360.
11. Bilgic B, Liao C, Manhard MK, et al. Robust high-quality multi-shot EPI with low-rank prior and machine learning. Proc Int Soc Magn Reson Med. 2019;1250.
Figures