0855
Nonpersistent broad-linewidth radicals generated using a 6 MeV linear electron accelerator for application in Dynamic Nuclear Polarization1Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, United Kingdom, 2GE Healthcare, Schenectady, NY, United States, 3Department of Chemistry and Photon Science Institute, University of Manchester, Manchester, United Kingdom, 4Centre for Advanced ESR, University of Oxford, Oxford, United Kingdom, 5MRC Oxford Institute for Radiation Oncology, University of Oxford, Oxford, United Kingdom, 6Department of Physics, University of Oxford, Oxford, United Kingdom, 7The MR Research Centre, Aarhus University, Aarhus, Denmark, 8Oxford Centre for Clinical Magnetic Resonance Research, John Radcliffe Hospital, Oxford, United Kingdom
Synopsis
Keywords: Hyperpolarized MR (Non-Gas), Contrast Agent
Stable free radicals formed in solid-state alanine (an established dosimeter) when exposed to ionizing radiation may be exploited to enable Dynamic Nuclear Polarization (DNP). Here, we used 6 MeV electron irradiation to generate nonpersistent radicals for the hyperpolarization of sterilized 13C-alanine. Electron Paramagnetic Resonance (EPR) confirmed radical formation, linear with dose up to 70 kGy. DNP build-up was demonstrated for solid 13C-alanine irradiated to 100 kGy. The broad EPR spectrum suggests the need for microwave frequency modulation to fully exploit the radiation induced nonpersistent radicals. However, this novel methodology makes DNP of solid substrates without a glassing agent possible.Introduction
Dynamic Nuclear Polarization (DNP) is an emerging technology for the production of hyperpolarized contrast agents (HCAs)1. Conventional DNP requires the introduction of chemically stabilized radical compounds to HCAs to provide a source of free electrons for spin exchange2. An emerging alternative for agents that can glass is to generate nonpersistent free radicals in situ using ultraviolet irradiation3.Here, we explore an alternative and novel method for the introduction of stable free electrons in situ using 6 MeV electron irradiation. 13C-alanine was chosen for this proof-of-concept study due to its demonstrated use as a dosimeter4 and the recombination of its generated radicals upon exposure to moisture5. Thus, it was hypothesised that these nonpersistent endogenous radicals could be used to hyperpolarize ¹³C-alanine without the need for filtering samples following DNP. In addition to lengthening the T1 of samples during their transfer between the polarizer and MR scanner, other potential benefits include: a radical concentration tuneable with irradiation dose and independent of toxicity concerns; flexibility of solvent choice; inherent sterilization of samples; and DNP of solid substrates without the addition of a glassing agent.
Methods
We explored multiple sample preparations utilising alanine. “Liquid state” DNP sample preparation was based on the protocol outlined by Hu et al6: 350 mg [1-13C]L-alanine (Sigma Aldrich), 250 µL 18.94 M NaOH, 100 μL DMSO, 15 mM OX063 trityl, and 0.3 mM Dotarem gadolinium (Guerbet) were mixed in the liquid state, snap-frozen and polarized. The replacement of OX063 trityl with either 15 mM TEMPO or irradiated [1-13C]L-alanine was also explored. [1-13C]L-alanine was irradiated using a 6 MeV high-dose rate electron linear accelerator (in-house developed, Oxford, UK)7. Secondly, “solid state” samples contained solely irradiated [1-13C]L-alanine powder with a frozen glycerol “plug” on top. These plugs were made by pipetting droplets of glycerol into liquid nitrogen. Another sample preparation comprised irradiated [1-13C]L-alanine powder mixed in anhydrous glycerol at room temperature.Each sample was pipetted into a small PEEK sample cup and inserted into a Hypersense polarizer (Oxford Instruments, Abingdon, UK) at 1.4 K, and 3.35 T/94 GHz for DNP. Frequency sweeps (93.750–94.195 GHz @ 100 mW, two minutes build-up per frequency) were acquired for each sample and used to determine the optimal frequency. Polarization build-up at this frequency was subsequently monitored every three minutes using a low flip-angle readout. The acquired spectra were analysed in jMRUI and normalized for the mass of 13C-alanine.
Continuous-wave electron paramagnetic resonance (EPR) spectra were collected to quantitively compare the number of radicals within each sample, and were recorded on a Bruker EMXMICRO X-band continuous wave spectrometer at room temperature.
Results
The generation of endogenous radical species upon 6 MeV electron irradiation of [1-13C]L-alanine was confirmed by an increase in spin count with irradiation dose (Figure 1). Each 10 kGy dose of irradiation was approximately equivalent to the addition of 10 mM TEMPO.Reflecting their short recombination time, the endogenous radicals did not appear to polarize “liquid state” 13C-alanine DNP samples, nor contribute to the spin count when prepared in the liquid state (Figure 2).
However, as expected, the opposite was observed from samples prepared in the solid state (Figure 3). An improvement on these signal enhancements was achieved by mixing irradiated 13C-alanine powder with glycerol (Figure 4A). The signal enhancement was also around a factor of 10 greater than that recorded from a liquid state sample preparation containing 15 mM TEMPO.
The greatest signal enhancement was recorded from the OX063 radical sample preparation replicated from the literature (Figure 4B). However, the highest spin count was recorded from the sample consisting of alanine powder mixed with glycerol (Figure 4C). From the EPR spectra (Figure 5) differences in the line shapes and widths between different radical species were observed. The endogenous radical EPR spectrum showed the presence of the hyperfine interaction between 13C and the electron and its line width was approximately three and eight times wider than that recorded from TEMPO and OX063, respectively.
Discussion
This work shows the potential for 13C-alanine to be polarized via nonpersistent radicals generated in situ by ionizing radiation. Of the methods tried, optimal polarization was reached when dry irradiated alanine powder was mixed with glycerol, which also maximized spin count. However, greater signal enhancement was observed from samples containing the exogenous radical, OX063 trityl. To elucidate the mechanisms underlying these results, further investigation is required.We propose that the continuous-microwave DNP scheme used here is interacting with only a proportion of spins present in a wider EPR line shape. Incorporating microwave frequency modulation may enable the saturation of a larger number of EPR transitions by engaging a larger fraction of the electron population. The success of this technique will depend on the correlation time/T1e of the radicals. However, their rapid recombination time post-dissolution is likely to improve the liquid-state nuclear T1 compared to the use of chemically stabilized radicals.
Conclusion
This work demonstrated the feasibility of polarizing 13C-alanine via nonpersistent radicals generated in situ by high energy electron irradiation. Potentially, this method could be used for the polarization of other solid agents which form persistent radicals in the solid state. To improve the signal enhancements generated here microwave frequency modulation will be explored.Acknowledgements
CHER would like to acknowledge the Engineering and Physical Sciences Research Council (EPSRC) and Medical Research Council (MRC) [grant number EP/L016052/1]. The authors would also like to acknowledge members of the Cardiac Metabolism Research Group (CMRG).References
[1] Pinon, A.C., Capozzi, A. & Ardenkjær-Larsen, J.H. (2021). Hyperpolarization via dissolution dynamic nuclear polarization: new technological and methodological advances. Magn Reson Mater Phy 34, 5–23. https://doi.org/10.1007/s10334-020-00894-w
[2] Wenckebach, W. T., & Quan, Y. (2021). Monte Carlo study of the spin-spin interactions between radicals used for dynamic nuclear polarization. Journal of Magnetic Resonance, 326, 106948.
[3] Capozzi, A., Hyacinthe, J. N., Cheng, T., Eichhorn, T. R., Boero, G., Roussel, C., ... & Comment, A. (2015). Photoinduced nonpersistent radicals as polarizing agents for X-nuclei dissolution dynamic nuclear polarization. The Journal of Physical Chemistry C, 119(39), 22632-22639.
[4] Bradshaw, W. W., Cadena, D. G., Crawford, G. W., & Spetzler, H. A. W. (1962). The use of alanine as a solid dosimeter. Radiation research, 17(1), 11-21.
[5] Sleptchonok, O. F., Nagy, V., & Desrosiers, M. F. (2000). Advancements in accuracy of the alanine dosimetry system. Part 1. The effects of environmental humidity. Radiation Physics and Chemistry, 57(2), 115-133.
[6] Hu, S., Zhu, M., Yoshihara, H. A., Wilson, D. M., Keshari, K. R., Shin, P., Reed, G., von Morze, C., Bok, R., Larson, P. E., Kurhanewicz, J., & Vigneron, D. B. (2011). In vivo measurement of normal rat intracellular pyruvate and lactate levels after injection of hyperpolarized [1-(13)C]alanine. Magnetic resonance imaging, 29(8), 1035–1040. https://doi.org/10.1016/j.mri.2011.07.001
[7] Berne, A., Petersson, K., Tullis, I. D., Newman, R. G., & Vojnovic, B. (2021). Monitoring electron energies during FLASH irradiations. Physics in Medicine & Biology, 66(4), 045015.
Figures
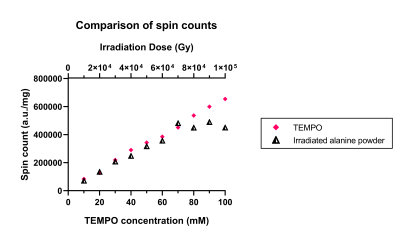
Figure 1: Confirmation of radical generation.
An increase in spin count with irradiation dose was recorded from irradiated alanine powder. Compared to samples containing different concentrations of TEMPO, up to 70 kGy, each additional 10 kGy dose of irradiation appeared to be approximately equivalent to adding 10 mM of the exogenous radical to the DNP sample preparation. This data confirmed the successful generation of radicals within alanine samples upon irradiation with 6 MeV electrons.
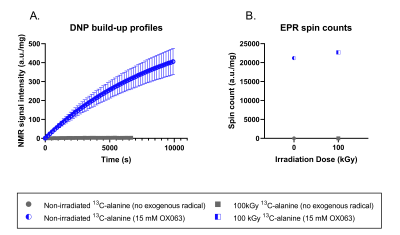
Figure 2: "Liquid state" Alanine DNP and EPR.
Build-up profiles showing little-to-no signal enhancement from the sample containing irradiated 13C-alanine (n=1) compared to samples containing the exogenous radical OX063 (n=3). Using EPR, only radicals from the exogenous radical compound appeared to contribute to the recorded B) spin count. Thus, in agreement with the literature for non-enriched alanine, the irradiation generated radicals in 13C-alanine rapidly recombine in the liquid state.
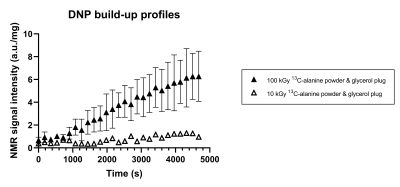
Figure 3: "Solid state" Alanine DNP and EPR.
Dot plot showing the build-up profiles recorded from "solid state" sample preparations containing 13C-alanine irradiated at different doses. Greater signal enhancement appeared to be recorded from the samples containing 13C-alanine irradiated at 100 kGy (n=3) compared to the sample irradiated at 10 kGy (n=1). Thus, in dry solid state alanine samples, the ionizing radiation generated radicals can be used to polarize 13C nuclei.
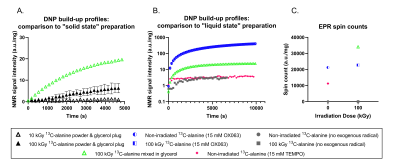
Figure 4.: Glycerol as a glassing matrix.
Compared to the A) DNP build-up profiles recorded from 13C-alanine powder samples, 100 kGy 13C-alanine mixed with glycerol appeared to show greater signal enhancements. This appeared to hold compared to some representative B) liquid-state samples, however, remained lower than the exogenous radical (OX063) sample preparation replicated from the literature. The increase in C) EPR spin counts, however, suggests only a fraction of the irradiation generated radicals are contributing to the polarization of 13C-alanine.
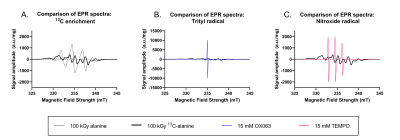
Figure 5: Comparison of EPR spectra.
Comparison of EPR line shapes and widths between 100 kGy irradiated ¹³C-alanine and A) 100 kGy irradiated non-enriched alanine, B) 15 mM OX063 and, C) 15 mM TEMPO. The difference in line shape compared to the non-enriched irradiated alanine is due to hyperfine coupling between ¹³C nuclei and the generated radicals. Irradiated samples had a wider line width than the exogenous radicals and hence may benefit from microwave frequency modulation during DNP.