0854
Over 20% 13C-Hyperpolarization and Fast Imaging of Ethyl-[1-13C]-Acetate-d6 and Ethyl-[1-13C]-Pyruvate-d6 using SAMBADENA1Division of Medical Physics, Department of Diagnostic and Interventional Radiology, University Medical Center Frieburg, Freiburg, Germany, 2German Cancer Research Center (DKFZ), Heidelberg, Germany, 3Department of Radiology and Neuroradiology, University Medical Center Schleswig-Holstein and Kiel University, Kiel, Germany, 4Otto Diels Institute for Organic Chemistry, Kiel University, Kiel, Germany, 5Chemistry, Wayne State Univeristy, Detroit, MI, United States, 6Russian Academy of Sciences (RAS), Moscow, Russian Federation
Synopsis
Keywords: High-Field MRI, Hyperpolarized MR (Non-Gas), Parahydrogen
Parahydrogen is a cost-efficient source of spin order for high-throughput production of hyperpolarized contrast agents. The parahydrogen approach SAMBADENA has shown great potential for biomedical applications: agents are polarized in situ in the MRI system at low cost with little additional hardware, repeatedly every 15s, and administration in vivo has been demonstrated. Here we present a new setup and high 13C polarization of 28% or 19% for ethyl-[1-13C]-acetate-d6 and ethyl-[1-13C]-pyruvate-d6, respectively, at concentrations of up to 80mM. We envision SAMBADENA to become a versatile method for the widespread application of hyperpolarized MRI and high throughput metabolic 13C MRI studies.Introduction:
Although 1H-MRI is very successful in medical diagnostics, the technique suffers from intrinsically low sensitivity.1 Hyperpolarization (HP) techniques have enabled signal enhancements of >10,000-fold for selected molecules and real-time imaging of metabolic conversion.2 Parahydrogen- (pH2) induced polarization (PHIP) is a cost- and time-efficient HP method and PHIP by side arm hydrogenation (PHIP-SAH) has enabled HP of pyruvate, acetate, and other metabolites.3,4 The PHIP method Synthesis Amid the Magnet Bore Allows Dramatically Enhanced Nuclear Alignment (SAMBADENA) in situ within the MRI system, fast administration, and 13C-imaging in vivo within seconds.5,6 Here, we demonstrate SAMBADENA of ethyl-[1-13C]-pyruvate-d6 (EP) and ethyl-[1-13C]-acetate-d6 (EA) in less than 8 seconds with 13C-polarizations as high as 20% and 30%, respectively. An in-house designed reactor that allows for increasing the temperature of the reaction to >70°C at 30 bar was key to achieving these results.Methods:
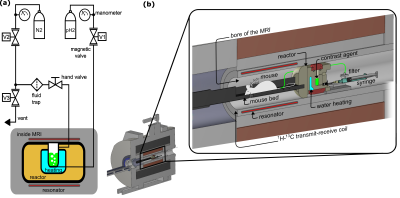
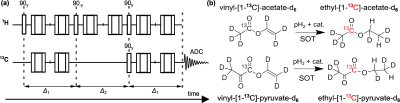
Setup: The SAMBADENA setup comprised a reactor custom-made from PEEK for fast pH2 addition at up to p=32 bar and T=90 °C, a fluid control unit, and the 7T preclinical MRI setup with 1H-13C volume coil (Fig. 1). The reactor was attached to a mouse bed and positioned in the isocenter of the magnet. The fluid control included a set of three magnetic valves, which were operated via a microcontroller-based relay box from the MRI pulse program, and one hand valve. For spin order transfer (SOT) from pH2-derived protons to 13C, the ESOTHERIC sequence was used with two composite refocusing pulses per interval (90°x-180°y-90°x, Fig. 2a).7 During the experiments, vinyl-[1-13C]-acetate-d6 (VA) was hydrogenated to ethyl-[1-13C]-acetate-d6 (EA, ≈1.1% naturally abundant 13C, precursor CAS: 189765-98-8) and vinyl-[1-13C]-pyruvate-d6 (VP) formed ethyl-[1-13C]-pyruvate-d6 (EP, ≈98% 13C enriched, synthesized by the Herges lab8).To commence the experiment, 1-mL acetone-d6 containing the PHIP-SAH precursors (Fig. 2b) and a rhodium-based catalyst was filled into the reactor (inside the MRI), pressurized to 5 bar N2 to avoid boil-off (from the top), and warmed using the reactor-integrated water heating. Then, the hand valve at the reactor outlet was closed. The pH2 addition was started by opening valve V1 for 7s to inject pressurized pH2 (≈80% enrichment) from the bottom of the reactor before the SOT sequence was started. Hence, a sample was hyperpolarized within ≈7.4s (i.e., 7s hydrogenation + 388ms SOT).
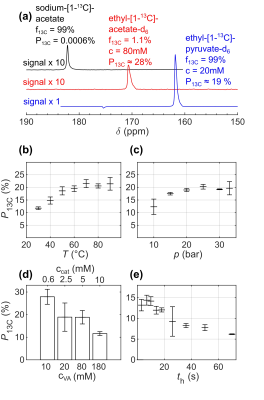
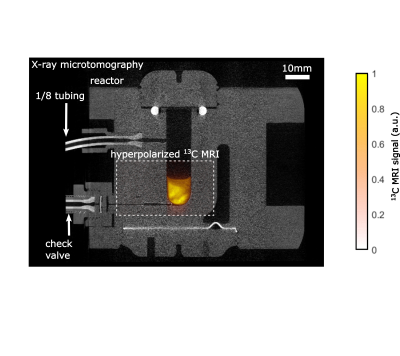
In most cases, the hyperpolarized 13C signal was detected at the end of the SOT. For 13C MRI, a 13C flip-back pulse was added to the SOT, and MRI acquisition was started subsequently (single-shot 13C-RARE, 128x64x1 matrix size, 40x24mm FOV, 15mm slice thickness, 0.31mm x 0.38mm in-plane resolution, 64 echoes, 438.6ms acquisition time). For coregistration, an X-ray micro-tomography (µCT) image of the empty reactor was acquired to depict the setup (res.: 20 µm, exposure: 266ms angular step: 0.8°, Bruker SkyScan 1276). 13C-HP was quantified assuming 100% hydrogenation yield and pH2 enrichment by comparing the HP signals with a thermally polarized reference solution (4 M [1-13C] sodium acetate in 1.5 mL H2O, 99 % 13C, Gd-doped, T1 = 832 ms).
Results:
High 13C-HP of 28% and 19% were detected for ethyl-[1-13C]-acetate-d6 (80mM, 5mM catalyst) and ethyl-[1-13C]-pyruvate-d6 (20mM, 5mM catalyst), respectively, at T=90°C and p=30bar pH2 pressure after 7s total hydrogenation time with only using 80% pH2 (Fig. 3). These results were preceded by extensive optimization and analysis using VA (Fig. 3) and would increase to ~40% and 26% 13C polarization, respectively if 100% pH2 were used. The elevated reaction temperature and pressure were found to be favorable to achieving high polarization of 80mM EA. We found that the maximum 13C HP of 80mM EA was observed after 7s hydrogenation and that relaxation of the pH2-derived 1H was dominating the reaction of residual PHIP-SAH precursor after this time (Fig. 3e). When the concentration of VA was increased from 10 to 160mM under otherwise identical conditions, we observed a gradual decrease of the polarization (Fig. 3d). Sub-second 13C MRI of 20mM hyperpolarized EP detected an intense 13C signal from the reaction chamber (Fig. 3).Discussion:
The high 13C-polarizations of relatively highly concentrated pyruvate and acetate precursors observed in situ here are very promising. If 100% enriched pH2 were used, the HP would increase further by a factor of ≈1.4. The decrease of polarization with increasing concentration (Fig. 3e) suggests incomplete hydrogenation for highly concentrated solutions. We expect that the yield can be improved by more efficient pH2 dissolution, e.g., using an H2 sparger or pH2-presaturated solution.10 To extract neat aqueous metabolite solutions for biomedical applications, fast side-arm cleavage, and solution purification need to be achieved. For this result, deuterium-labeled PHIP-SAH precursors were essential, which became available only recently.8 To this end, we are currently working on implementing methods from the literature into our current protocol.10,11Conclusion:
Efficient SAMBADENA of PHIP-SAH precursors of acetate and pyruvate was demonstrated and may likely be improved further along with improved pH2 dissolution and enrichment. The polarizations and concentrations observed are approaching the numbers needed for in vivo imaging, although purification was not attempted. Considering the low (additional) cost, small footprint,12 high sample throughput,6 and fast in vivo administration,5 SAMBADENA holds great promise to accelerate the translation of PHIP to lower the translational burden and enable widespread use of HP for metabolic MRI.Acknowledgements
This work was supported by the German Cancer Consortium (DKTK), B.E.S.T. Fluidsysteme GmbH I Swagelok Stuttgart, the German Research Foundation (SCHM 3694/1-1, SCHM 3694/2-1, SFB1479, HO-4604/2, HO-4604/3), and the Federal Ministry of Education and Research (BMBF) lighthouse project QuE-MRT and Juniorverbund 01ZX1915C (AP). ABS and EYC thank Wayne State University for Postdoctoral Fellow award.References
1. Asch, F. M. et al. Lack of sensitivity of the electrocardiogram for detection of old myocardial infarction: A cardiac magnetic resonance imaging study. American Heart Journal 152, 742–748 (2006).
2. Chowdhury, R. et al. Quantification of Prostate Cancer Metabolism Using 3D Multiecho bSSFP and Hyperpolarized [ 1‐ 13C] Pyruvate: Metabolism Differs Between Tumors of the Same Gleason Grade. Magnetic Resonance Imaging jmri.28467 (2022) doi:10.1002/jmri.28467.
3. Reineri, F., Boi, T. & Aime, S. ParaHydrogen Induced Polarization of 13C carboxylate resonance in acetate and pyruvate. Nat Commun 6, 5858 (2015).
4. Schmidt, A. B. et al. Instrumentation for Hydrogenative Parahydrogen-Based Hyperpolarization Techniques. Anal. Chem. 94, 479–502 (2022).
5. Schmidt, A. B. In vivo 13C-MRI using SAMBADENA. (2018).
6. Schmidt, A. B. et al. Quasi-continuous production of highly hyperpolarized carbon-13 contrast agents every 15 seconds within an MRI system. Commun Chem 5, 21 (2022).
7. Korchak, S., Mamone, S. & Glöggler, S. Over 50 % 1H and 13C Polarization for Generating Hyperpolarized Metabolites—A parr‐Hydrogen Approach. ChemistryOpen 7, 672–676 (2018).
8. Brahms, A. et al. Synthesis of 13C and 2H Labeled Vinyl Pyruvate and Hyperpolarization of Pyruvate. Chemistry A European J 28, (2022).
9. Korchak, S., Yang, S., Mamone, S. & Glöggler, S. Pulsed Magnetic Resonance to Signal-Enhance Metabolites within Seconds by utilizing para-Hydrogen. ChemistryOpen 7, 344–348 (2018).
10. Knecht, S. et al. Rapid hyperpolarization and purification of the metabolite fumarate in aqueous solution. Proc. Natl. Acad. Sci. U.S.A. 118, e2025383118 (2021).
11. Hune, T. et al. Metabolic Tumor Imaging with Rapidly Signal‐Enhanced 1‐ 13C‐Pyruvate‐d3. ChemPhysChem (2022) doi:10.1002/cphc.202200615.
12. Schmidt, A. B. et al. Liquid-state carbon-13 hyperpolarization generated in an MRI system for fast imaging. Nat Commun 8, 14535 (2017).
Figures