0853
First in vivo experiences with [1-13C]-pyruvate hyperpolarized by SABRE1Chemistry, North Carolina State University, Raleigh, NC, United States, 2Vizma Life Sciences, Raleigh, NC, United States, 3Chemistry, Southern Illinois University, Carbondale, IL, United States, 4A. A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 5Chemistry, Wayne State University, Detroit, MI, United States
Synopsis
Keywords: Hybrid & Novel Systems Technology, Hyperpolarized MR (Non-Gas)
We present the first in vivo hyperpolarized MR using Signal Amplification By Reversible Exchange.Introduction
The presented work was conducted to demonstrate the feasibility of in vivo hyperpolarized detection of metabolism using SABRE. SABRE is a parahydrogen induced polarization (PHIP) technique that was first described in 2009.1 The field of SABRE has advanced substantially since then, yet to the best of our knowledge, SABRE has not been translated to living animal models. In the course of SABRE development, significant milestones included the direct hyperpolarization of heteronuclei described in 2015,2,3 which enabled the hyperpolarization of [1-13C]pyruvate,4 an approach that was improved more recently to produce up to 15% polarization on [1-13C]pyruvate5,6,7—setting the stage for pilot in vivo demonstrations. The use of SABRE for hyperpolarization is attractive because it is rapid and uses relatively simple instrumentation. Moreover, SABRE hyperpolarizes the substrate directly, thereby circumventing the need for difficult-to-make precursors, chemical steps (i.e. hydrolysis), or neutralization to obtain hyperpolarized [1-13C]pyruvate.Methods
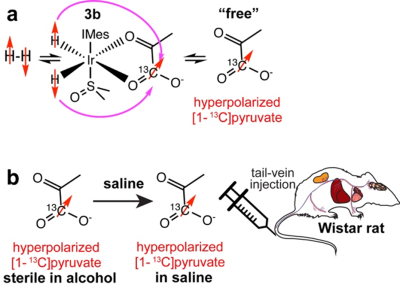
Hyperpolarized sodium [1-13C]pyruvate was produced in a method similar to that reported recently.5 In brief, a solution of 6 mM [Ir(COD) IMes Cl] where IMes = 1,3 ‐ bis(2,4,6 ‐ trimethylphenyl) imidazole‐2‐ylidene, 65 mM [1-13C]pyruvate, 24 mM DMSO in 0.5 mL methanol is activated by bubbling parahydrogen through the solution at room temperature, which leads to the formation of the active hyperpolarization transfer catalyst Ir(H)2(η2-[1-13C]pyruvate)(DMSO)(IMes), displayed as 3b Figure 1a. The activated solution is cooled to 0 °C and then placed inside a 0.5 µT magnetic field, where parahydrogen is bubbled through the solution for 60 s. During this process, the sample warms up and yields approximately 10% polarization of the free [1-13C]pyruvate in solution. Subsequently, the sample is placed in a Halbach array of 0.3 T and the hydrogen pressure is released. The solution is pulled into a syringe containing 1 mL of aqueous saline such that the methanol:water ratio is 1:2, still containing Ir-catalyst. This mixture was then administered into the tail vein of the anesthetized Wistar rat, Figure 1b. In this feasibility study, all experiments were terminal in that the animals were euthanized before waking from anesthesia. These experiments were limited to a small number of animals and approved by the IACUCs at NC State University and Massachusetts General Hospital. After administration of the hyperpolarized [1-13C]pyruvate solution, the vitals (respiration rate, heart rate and temperature) of the anesthetized animals were stable and the hyperpolarized metabolites were observed spectroscopically with 20° or 30° pulses and TR of 2 or 3 seconds. At MGH a 13C surface coil was placed over the rat’s liver inside a 4.7 T pre-clinical MRI scanner. At NC State, a whole-body 13C volume coil was employed to record 13C NMR spectra over a whole rat body at 1.5 T.Results
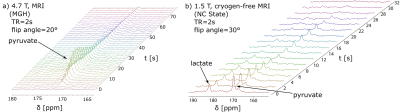
The resulting 13C spectroscopic data is displayed in Figure 1. Figure 1a shows hyperpolarized [1-13C]pyruvate signal build-up and decay as a function of time as the hyperpolarized bolus enters the active region of the 13C surface coil placed on the rat liver, followed by decay through T1 relaxation and metabolism. The 13C hyperpolarized signal remained detectable for over one minute. Figure 1b shows a hyperpolarized spectrum measuring the conversion of pyruvate to lactate across the whole body of a healthy animal, as this data was acquired with a 13C volume coil of a cryogen-free MRI system operated at 1.5 T.Discussion
To the best of our knowledge, the presented data are the first examples of any SABRE hyperpolarized substrate detected in vivo. The presented experiments still suffer from a significant number of imperfections that we expect to address in the near future. For example, the data displayed in Fig. 1a was obtained using uncertain parahydrogen enrichment because the parahydrogen was shipped from NC State University to Massachusetts General Hospital, which was significantly delayed, resulting in reduction of the parahydrogen fraction (estimated to be less than 50% at the time of experiment). In contrast, the experiments displayed in Figure 1b used 95% parahydrogen, however significant challenges in the administration of the hyperpolarized pyruvate led to non-adiabatic spin transport, which resulted in erasing close to 99% of our signal according to our current estimates.Conclusion
The field of SABRE has been awaiting the first demonstrations of in-vivo feasibility for over a decade. Here, we demonstrate that [1-13C]pyruvate can be readily hyperpolarized to above 10% with SABRE when controlling critical parameters of the hyperpolarization process including sample composition, temperature, and the magnetic field. These in vitro demonstrations encouraged us to attempt pilot in vivo experiments, which were immediately successful. While the current approach suffers from several shortcomings, many are readily addressable (e.g. using ~100% parahydrogen, avoiding non-adiabatic injection procedures, catalyst removal by filtration). More challenging is the transition to a fully biocompatible injectable solution that is free of methanol.8 Our current efforts are directly focused on overcoming these problems. Despite these current challenges, overall, the current experiments indicate that SABRE has a clear path to become a competitive hyperpolarization modality that shines in its simplicity and speed and can be combined with cryogen-free MRI to establish a simple, portable, and cost-effective molecular imaging platform in the future.Acknowledgements
This work was supported by NSF CHE-1905341 and CHE-1904780, NIBIB R21EB025313 and R01EB029829, and NIGMS R21GM137227. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. T.T. acknowledges funding from the North Carolina Biotechnology Center and the Mallinckrodt Foundation . MSR acknowledges the support of the Kiyomi and Ed Baird MGH Research Scholar award.References
1. Adams, R. W. et al. Reversible interactions with para-hydrogen enhance NMR sensitivity by polarization transfer. Science 323, 1708–1711 (2009).
2. Truong, M. L. et al. 15N Hyperpolarization by Reversible Exchange Using SABRE-SHEATH. J. Phys. Chem. C 119, 8786–8797 (2015).
3. Theis, T. et al. Microtesla SABRE Enables 10% Nitrogen-15 Nuclear Spin Polarization. J. Am. Chem. Soc. 137, 1404–1407 (2015).
4. Iali, W. et al. Hyperpolarising Pyruvate through Signal Amplification by Reversible Exchange (SABRE). Angew. Chemie - Int. Ed. 58, 10271–10275 (2019).
5. Tomhon, P. et al. Temperature Cycling Enables Efficient 13C SABRE-SHEATH Hyperpolarization and Imaging of [1-13C]-Pyruvate. J. Am. Chem. Soc. 144, 282–287 (2022).
6. Adelabu, I. et al. Order-Unity 13C Nuclear Polarization of [1-13C]Pyruvate in Seconds and the Interplay of Water and SABRE Enhancement. ChemPhysChem 23, e202100839 (2022).
7. Nantogma, S. et al. Interplay of Near-Zero-Field Dephasing, Rephasing, and Relaxation Dynamics and [1-13C]Pyruvate Polarization Transfer Efficiency in Pulsed SABRE-SHEATH. J. Phys. Chem. A jp-2022-07150b (2022).
8. Schmidt, A. et al. Catalyst-Free Aqueous Hyperpolarized [1-13C]Pyruvate Obtained by Re-Dissolution Signal Amplification by Reversible Exchange. ACS Sensors se-2022-01715p (2022).
Figures