0852
High-throughput kinetic analysis in a microfluidic multiwell platform by dissolution dynamic nuclear polarization-magnetic resonance
Marc Azagra1, Jose Yeste Lozano1, Maria Alejandra Ortega 1, Alejandro Ernesto Portela1, Gergö Matajsz1, Alba Herrero Gómez1, Kim Yaewon2, Renuka Sriram2, John Kurhanewicz2, Daniel Vigneron2, and Irene Marco Rius1
1MIPMED, IBEC, Barcelona, Spain, 2UCSF, San Francisco, CA, United States
1MIPMED, IBEC, Barcelona, Spain, 2UCSF, San Francisco, CA, United States
Synopsis
Keywords: Hyperpolarized MR (Non-Gas), New Devices, dissolution DNP, MRSI, microfluidics, multisample measurement
High throughput dissolution Dynamic Nuclear Polarization (dDNP) magnetic resonance spectroscopic imaging (MRSI) was achieved by developing a multiwell microfluidic platform. This platform allows for multiple experiments to be performed with a single injection of a dDNP substrate, enabling faster and more reproducible data gathering, reducing experimental costs and time by a factor of 8. Here, we present a proof of concept of the methodology with an oxidation reaction combining [1-13C]pyruvic acid with hydrogen peroxide, testing 2 different conditions with 3 replicates each.Introduction
Current dissolution dynamic nuclear polarisation (DNP) protocols are limited to a single sample measurement per dissolution process1. The rapid growth of hyperpolarization-enhanced technologies requires high-throughput methods to increase the yield and reproducibility of the analyses, as well as a substantial decrease of time and costs associated with DNP-MR experiments. In this work we demonstrate that the application of microfluidics can effectively enhance the throughput of one single DNP shot. We developed a microfluidic multiwell chip containing eight fluidic chambers that can be simultaneously measured upon injection of a hyperpolarized solution (Fig. 1). Hence, experimental variability between samples is minimized. To demonstrate its capabilities, DNP-MR spectroscopic images of the microfluidic platform were acquired to investigate the reaction kinetics and mechanisms of pyruvate peroxidation. In a single experiment, five control samples and three peroxidation reactions were tested simultaneously, including sampling of the temporal evolution of the reagents. Our proposed platform improves the DNP throughput by a factor of 8 for the study of chemical or biochemical reactions.Methods
A microfluidic multiwell plate made of three polydimethylsiloxane (PDMS) layers on a glass slide (2947-75X38, Corning) was designed and fabricated to allow for the infusion and withdrawal of solutions into 8 fluidic chambers (i.e. wells). These infusion and withdrawal channels were grouped in 2 independent sets of 4 wells that share the perfusion system (enabling 2 different conditions and 4 replicates). High-throughput with DNP-MRSI was probed by 13C-spectroscopic chemical shift imaging (CSI) upon injection of hyperpolarized [1-13C]pyruvic acid. All measurements were performed on a horizontal 3T scanner (BioSpec 105 mm bore diameter, Bruker) equipped with a dual-tunned 1H-13C volume coil (42 mm inner diameter, Bruker). The CSI spectra were acquired 10 s after the dissolution of 24 µL [1-13C]pyruvic acid (Sigma Aldrich, Munich, Germany) containing 15 mM trityl radical OX063 (GE healthcare) and 1.5 mM Dotarem (Guerbet, Villepinte, France).The localizer of the DNP-MRSI experiments was performed with axial, sagittal and coronal T2-weighted images using a spin echo T2 TurboRare sequence to assess signal location to program accurately the CSI voxels matrix. The CSI sequence was designed as an 8 x 8 matrix, with a field of view of 40 cm x 20 cm and a slice thickness of 12 mm, 15º flip angle, echo time = 1.49 ms, acquisition time = 51.2 ms and recycle time = 66.907 ms. Resulting data were reconstructed and processed in SIVIC2.Results
A microfluidic chip was designed, fabricated, and fully tested, obtaining a versatile platform to be applied in DNP-MRSI protocols. The multiwell platform allows a fast injection of 1.4 mL in 4 seconds.Figure 2 represents schematically how the hyperpolarized solution is injected in the microfluidic device, displays the 3 planes of the T2 weighted proton localizer images (axial, sagittal, and coronal) (Fig 2. B) and shows a representative spectrum of a 13C-MRS measurement from one voxel containing just hyperpolarized [1-13C]pyruvic acid (Fig 2. C). As a proof of concept for detecting fast reactions, we traced the 13C resonance shifts of the reaction between [1-13C]pyruvic acid and hydrogen peroxide, obtaining 13CO2 and two intermediates: [1-13C] 2 -hydroxyperoxy-2-hydroxypropanoate and [1-13C] peroxymonocarbonate.Discussion
After injection of the hyperpolarized pyruvate solution, the spectra of the chip regions without hydrogen peroxide displayed only the pyruvate peak because no reaction had occurred (Fig. 3). The rest of the voxels, in which the reaction took place, presented a characteristic multi-peak spectrum corresponding to the peroxidation reaction of the pyruvate (Fig. 4). Three resonance frequencies were identified on those spectra in addition to the C1 pyruvate and C1 pyruvate hydrate peaks. The additional peaks accounts for one intermediate and two subproducts of the reaction3. The signal decay along the time of the reagents, intermediates and products is shown in Figure 5.Conclusion
Hyperpolarization-enhanced MR techniques, such as DNP-NMR, are rapidly evolving and are expected to transform current analytical tools. Increasing their throughput will overcome the barrier that prevents the wider accessibility of these techniques. Here, we showed that combining DNP-MRSI and microfluidics can unprecedentedly increase the capacity of tracing chemical reactions and demonstrated that multiple samples can be simultaneously interrogated. This methodology could potentially be used to probe in-situ metabolomic data in biological cultures.Acknowledgements
We thank the MicroFabSpace and Microscopy Characterization Facility, Unit 7 of ICTS “NANBIOSIS” from CIBER-BBN at IBEC. We also thank Dr. Xiao Ji (UCSF), Christoffer Laustsen (Aarhus University, Denmark), Lotte Bonde Bertelsen (Aarhus University, Denmark), Nichlas Vous Christensen (Aarhus University, Denmark) for experimental assistance. This work has been possible thanks to the financial support through the Junior Leader Postdoctoral Fellowship Programme from “la Caixa” Banking Foundation (LCF/BQ/ PI18/11630020), Spanish MINECO project MCIN/AEI/10.13039/501100011033 (Ref. EIN2020-112209), BIST-“la Caixa” Banking Foundation Chemical Biology programme, AHG received financial support through the FI Fellowship Programme from AGAUR (Ref. 2021 FI_B_01039). the European Union “NextGenerationEU”/PRTR and the European Union's Horizon 2020 (FET Open) under grant agreement GA-863037 (BLOC).References
1. Marco-Rius I, Tayler MCD, Kettunen MI, et al. Hyperpolarized singlet lifetimes of pyruvate in human blood and in the mouse. NMR Biomed. 2013;26(12):1696-1704.2.
2. Crane JC, Olson MP, Nelson SJ. SIVIC: Open-Source, Standards-Based Software for DICOM MR Spectroscopy Workflows. Int J Biomed Imaging. 2013;2013.3.
3. Drachman N, Kadlecek S, Duncan I, Rizi R. Quantifying reaction kinetics of the non-enzymatic decarboxylation of pyruvate and production of peroxymonocarbonate with hyperpolarized 13C-NMR. Physical Chemistry Chemical Physics. 2017;19(29):19316-19325.
Figures
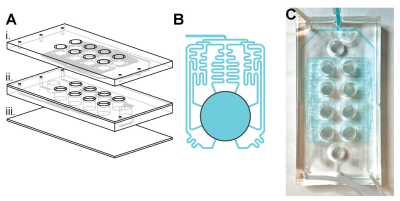
Figure 1. Microfluidic multiwell plate for high-throughput DNP-MRSI experiments. (A) Assembling layers of the multiwell plate. (B) Network of microfluidic channels that injects the polarized sample into one of the wells. This design is replicated along all the wells. (C) Photo of the microfluidic device. Microfluidic channels that distribute the polarized sample are filled with blue dye for easy identification.

Figure 2. High-throughput DNP-MRSI assay. (A) Schematic illustration of the experimental setup consisted of polarizer equipment, microfluidic multiwell plate, and magnetic resonance imaging scanner. (B) T2-weighted localizer images with axial, sagittal and coronal perspectives. (C) Stacked dynamic spectra of [1-13C]pyruvic acid along the experiment. Note that the first spectrum was acquired at 25 seconds after dissolution.
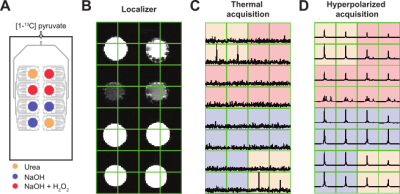
Figure 3. (A) Schematic image of the microfluidic device specifying the sample placed in each well. (B) Localizer with a coronal T2-weighted image with the corresponding voxels matrix overlapping. (C) Spectroscopic acquisition using a CSI pulse sequence to the microfluidic device at thermal equilibrium. (D) First spectroscopic acquisition point after the hyperpolarized [1‑13C]pyruvate injection.
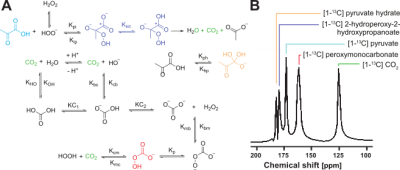
Figure 4. (A) Scheme of the oxidation reaction between pyruvic acid and hydrogen peroxide (This figure was adapted from Drachman et al.3) . (B) Representative spectrum of the decarboxylation of [1-13C]pyruvate reacted with H2O2.
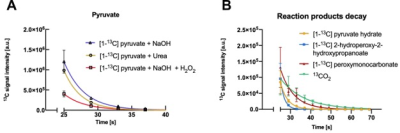
Figure 5. (A) Plot showing the signal decay of [1-13C]pyruvate from the wells containing [13C]urea (orange), the wells containing hydrogen peroxide and sodium hydroxide solution (red) and the wells containing water and sodium hydroxide solution (purple). (B) Plot showing the signal decay of [1-13C]pyruvate hydrate, [1-13C] 2-hydroperoxy-2-hydroxypropanoate, [1-13C] peroxymonocarbonate, and 13CO2 over time. The integral displayed in each all four voxels corresponding to a well were summed up and each well correspond to a replicate.
DOI: https://doi.org/10.58530/2023/0852