0846
Beyond lactate: using hyperpolarized [1-13C] pyruvate to measure human brain pH and amino acid metabolism1Department of Radiology, University of Cambridge, Cambridge, United Kingdom, 2Cancer Research UK Cambridge Institute, Cambridge, United Kingdom, 3GE Healthcare, GE Healthcare, Munich, Germany
Synopsis
Keywords: Hyperpolarized MR (Non-Gas), Hyperpolarized MR (Non-Gas)
Hyperpolarized 13C MRS was used to measure human brain pH for the first time following 13C imaging through measurements of 13CO2 and bicarbonate. Aspartate concentration was also found offering insight into amino acid metabolism. Global brain pH was measured in the brain to be 6.98 ± 0.06. Non-invasive measurements of cerebral pH could be particularly important in assessing cerebral pathology given the wide range of disease processes that alter acid-base balance. Aspartate was measured in 4 of 9 volunteers with no age-related trends seen. Pyruvate carboxylase flux was found to be 6% of pyruvate dehydrogenase flux.
Introduction
Hyperpolarized carbon-13 (HP 13C) MRI following injection of [1-13C]pyruvate is a metabolic imaging method that can measure tissue uptake of pyruvate and its metabolic conversion to downstream metabolic products. Conventionally, brain imaging studies have probed the metabolic conversion of pyruvate to lactate and bicarbonate1,2,3, however significant signals from other metabolites can also be measured in spectra acquired at the end of an imaging time course. Measurements of 13CO2 alongside bicarbonate can be used to estimate whole-brain pH through the Henderson Hasselbalch equation4. Additionally, the measurement of aspartate offers the ability to investigate flux through pyruvate carboxylase (PC): previous investigations have shown that aspartate labelling within the brain may be age related5. Since pyruvate dehydrogenase (PDH) activity is assumed to correlate with bicarbonate labelling, measurement of aspartate and bicarbonate can allow for the estimation of PC flux relative to PDH6.Methods
13C-MRI was performed in 13 volunteers using a dual-tuned 13C/1H birdcage head coil (Rapid Biomedical, Rimpar, Germany). [1-13C]Pyruvate was hyperpolarized using a SpinLab hyperpolarizer (GE Healthcare). Unlocalized 13C-MRS was acquired in 9 volunteers for the measurement of global pH, with sufficiently large RF excitation and acquisition bandwidths in order to excite and detect both H13CO3- and 13CO2 , (TR = 1 s, flip angle = 45°, 15000 Hz, 4096 points, pw = 0.25 ms, 44-49 seconds after injection). Slice selective 13C-MRS using either three or five slices was acquired in nine volunteers (6 from the same cohort as the pH measurements) to allow for the detection of aspartate (TR = 1 s, flip angle = 90°, 15000 Hz, 4096 points, 3-5 slices). Peak areas were integrated from spectra with first-order phase and baseline correction by two independent users. pH was calculated from the H13CO3- and 13CO2 peak areas using:$$pH=pK_a+log (\frac{H^{13}C O_3^-}{^{13}C O_2})$$
A pKa of 6 .17 was used for this experiment. The ratio of 13C-aspartate and H13CO3- in the central slice was used to infer the relative fluxes of PC and PDH. An aspartate SNR of 2.5 was set as the limit for detection.
Results
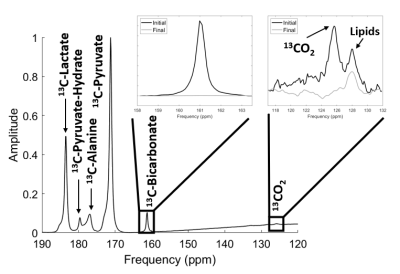
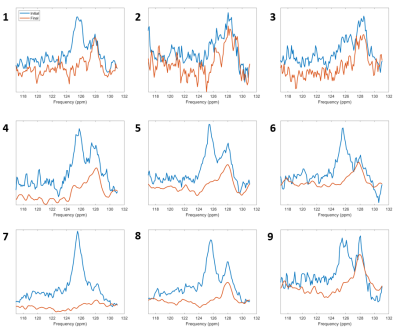
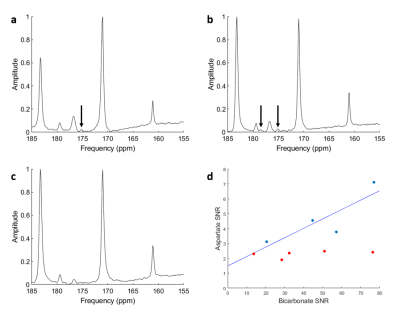
A non-localized 13C spectrum is shown in figure 1, with 13CO2 (125.6 ppm) and H13CO3- (161 ppm) peaks used for the calculation of pH shown. The hyperpolarized 13CO2 peak was verified by measuring the decay of the signal over multiple excitations, leaving only the lipid peaks remaining. 13CO2 and therefore pH was measured in 7/9 cases. The pH measurements calculated by two users are shown in table 1, with spectra from all volunteers shown in figure 2.Aspartate was measured in 4/9 volunteers. Figure 3 shows the spectra from three volunteers of various ages, with aspartate being detected in both younger and older subjects but was not detected in a third case of a younger age volunteer despite spectra with similar SNR. In one case (volunteer #12) [4-13C] Aspartate was also seen. Pyruvate carboxylase flux was inferred to be around 6% of PDH flux from a linear fit of aspartate against bicarbonate of y = 0.063x + 1.493. Table 2 details the SNR levels of pyruvate, lactate and bicarbonate for the nine volunteers
Discussion
The pH was measured in 7/9 volunteers for the first time using HP 13C pyruvate in the brain. pH measurements were in agreement with physiological predictions and with good inter-rater reproducibility (0.07 pH units) and CoV (1.6%). The need for the large bandwidth and SNR of unlocalized MRS restricts the pH measurement to global brain values. Since bicarbonate is produced within the intracellular space, the intracellular compartment is expected to dominate the pH value, however there may be some extracellular contributions due to diffusion into the extracellular space. The failure to detect CO2 in two cases due to polarization below 10 % highlighted the technical requirements for detection with > 10 % polarisation having to remain for the signal to be detectable. CO2 measurement, and therefore pH estimation, has been shown to be feasible in the heart (where bicarbonate production is greater) in both animal and human studies7,8. Whilst pH measurements using 31P and HP-13C bicarbonate9,10 are established, they are limited by clinical translation and technical requirements. The ability to use HP-13C pyruvate to measures pH offers an additional metric to an imaging technique that has already been demonstrated to have clinical translation.Whilst previous studies5 have found aspartate production to decline with age, in the four volunteers in which aspartate was seen there was no relationship with age. The relative signals of aspartate and bicarbonate suggested a PC flux of about 6% of the PDH flux, in agreement with previous literature6. Both pH and PC/PDH flux changes may provide interesting measures in tumours and other brain pathologies, at diagnosis and in response to treatment.
Conclusion
This study demonstrates that using unlocalized and slice-selective 13C-MRS of the brain, acquired at the end of an imaging time course, allows the estimation of global pH and PC/PDH flux. This offers the potential to assess vital cerebral markers.Acknowledgements
We acknowledge support from CRUK, CRUK Cambridge Centre, NIHR Cambridge BRC, NIHR Cambridge Clinical Research Facility, Addenbrooke’s charitable Trust, the Evelyn Trust, Cambridge Experimental Cancer Medicine Centre, Lundbeck Foundation and the MS Society.References
1) Zaccagna, F., McLean, M.A., Grist, J.T., Kaggie, J., Mair, R., Riemer, F., Woitek, R., Gill, A.B., Deen, S., Daniels, C.J. and Ursprung, S., 2022. Imaging Glioblastoma Metabolism by Using Hyperpolarized [1-13C] Pyruvate Demonstrates Heterogeneity in Lactate Labelling: A Proof of Principle Study. Radiology: Imaging Cancer, 4(4), 210076.
2) Nelson, S.J., Kurhanewicz, J., Vigneron, D.B., Larson, P.E., Harzstark, A.L., Ferrone, M., Van Criekinge, M., Chang, J.W., Bok, R., Park, I. and Reed, G., 2013. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C] pyruvate. Science translational medicine, 5(198).
3) Miloushev, V.Z., Granlund, K.L., Boltyanskiy, R., Lyashchenko, S.K., DeAngelis, L.M., Mellinghoff, I.K., Brennan, C.W., Tabar, V., Yang, T.J., Holodny, A.I. and Sosa, R.E., 2018. Metabolic Imaging of the Human Brain with Hyperpolarized 13C Pyruvate Demonstrates 13C Lactate Production in Brain Tumor PatientsHyperpolarized MRI of the Human Brain. Cancer research, 78(14), pp.3755-3760
4) Gallagher, F.A., Kettunen, M.I. and Brindle, K.M., 2011. Imaging pH with hyperpolarized 13C. NMR in biomedicine, 24(8), 1006-1015.
5) Chen, A.P., Lee, C.Y., Geraghty, B.J., Perks, W.J., Soliman, H. and Cunningham, C.H., Detection of pyruvate carboxylation and age related changes in glycolysis in human brain using hyperpolarized 13C MR spectroscopy. Proc. Intl. Soc. Mag. Reson. Med. 27 (2019), 4301.
6) Mason, G.F., Petersen, K.F., De Graaf, R.A., Shulman, G.I. and Rothman, D.L., 2007. Measurements of the anaplerotic rate in the human cerebral cortex using 13C magnetic resonance spectroscopy and [1‐13C] and [2‐13C] glucose. Journal of neurochemistry, 100(1), 73-86.
7) Moon, R.B. and Richards, J.H., 1973. Determination of intracellular pH by 31P magnetic resonance. Journal of Biological Chemistry, 248(20), 7276-7278.
8) Cunningham, C.H., Lau, J.Y., Chen, A.P., Geraghty, B.J., Perks, W.J., Roifman, I., Wright, G.A. and Connelly, K.A., 2016. Hyperpolarized 13C metabolic MRI of the human heart: initial experience. Circulation research, 119(11), 1177-1182.
9) Gallagher, F.A., Kettunen, M.I., Day, S.E., Hu, D.E., Ardenkjær-Larsen, J.H., Zandt, R., Jensen, P.R., Karlsson, M., Golman, K., Lerche, M.H. and Brindle, K.M., 2008. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature, 453(7197), 940-943.
10) Schroeder, M.A., Swietach, P., Atherton, H.J., Gallagher, F.A., Lee, P., Radda, G.K., Clarke, K. and Tyler, D.J., 2010. Measuring intracellular pH in the heart using hyperpolarized carbon dioxide and bicarbonate: a 13C and 31P magnetic resonance spectroscopy
Figures