0844
PDH flux is a sensitive biomarker of mitochondrial dysfunction in acute TBI1Advanced Imaging Research Center, UT Southwestern Medical Center at Dallas, Dallas, TX, United States, 2Department of Neurology and Neurotherapeutics, UT Southwestern Medical Center at Dallas, Dallas, TX, United States, 3Radiology, Loma Linda University, Loma Linda, CA, United States, 4Advanced Imaging Research Center,, UT Southwestern Medical Center at Dallas, Dallas, TX, United States, 5Department of Radiology, UT Southwestern Medical Center at Dallas, Dallas, TX, United States
Synopsis
Keywords: Hyperpolarized MR (Non-Gas), Brain
Due to the rapid and complex progression of metabolic alteration after traumatic brain injury (TBI), prompt assessment of cerebral metabolism is critical in preventing the subsequent injury processes and developing proper therapeutic strategies. In this study, we assessed longitudinal changes in bicarbonate production from hyperpolarized [1-13C]pyruvate in a rat TBI model. In addition to elevated lactate production, we observed significantly reduced bicarbonate production in the injured site. The contrast of bicarbonate signals between the injured region and the contralateral brain peaked at one day post-injury. A subset of TBI rats demonstrated markedly increased bicarbonate in contralateral brain regions post-injury.Introduction
TBI contributes to approximately 30% of all injury-related deaths in the United States with over 50 % of survivors sustaining long-term disability1-3. A major challenge of treating patients with TBI are the compounding secondary injury effects following the primary mechanical damage. Understanding the timing of secondary TBI damage can provide precise windows of therapeutic intervention to prevent or reduce subsequent injuries, thus directly impacting long-term patient outcome. While a number of pathological alterations in TBI are potential biomarkers, no current clinical imaging modalities are sensitive enough to routinely resolve the details of metabolic shifts in different brain sub-regions with secondary injury. Therefore, an appreciation of the chronological metabolic changes that occur after TBI may help provide insight into these secondary impairments. Combination of dynamic nuclear polarization (DNP) of 13C-labeled substrates, which raise the signal-to-noise ratio (SNR), and 13C MR spectroscopy imaging provides unique non-invasive measurements of critical in-vivo dynamic metabolic processes. In particular, hyperpolarized [1-13C]pyruvate has been used to assess lactate dehydrogenase (LDH) and pyruvate dehydrogenase (PDH) activities in the brain. Previously, metabolic imaging studies with hyperpolarized [1-13C]pyruvate reported increased lactate production in the damaged brain region following a controlled cortical impact (CCI) rat model up to seven days4. In this study, we assessed the chronology of bicarbonate production using hyperpolarized [1-13C]pyruvate in rodents after a CCI.Methods
Animal: Male Wistar rats were split into 2 groups: TBI model (n = 31) induced by CCI according to the protocol5 or Sham (n = 33) control. Sample preparation and MR protocol: 14-M [1-13C]-labeled pyruvic acid (MilliporeSigma) and 15-mM trityl radical OX063 (Oxford Instrument) was polarized using a SPINlab polarizer (GE Healthcare). All imaging experiments were performed on a clinical 3T MRI Scanner (GE Healthcare). For both radiofrequency (RF) excitation and signal reception, a 13C/1H dual-tuned quadrature birdcage rat coil (GE Healthcare) was used. After a three-plane localizer scan, 2D T2-weighted dual-echo fast spin echo images were acquired for anatomical reference to localize the sites of injury. For 13C metabolic imaging, each rat was given a bolus of hyperpolarized [1-13C]pyruvate (0.8 mmol/kg body weight) at a rate of 0.25 mL/s, and images were acquired with a phase-encoded free-induction decay chemical shift imaging sequence with a single axial slice centered on the injury site. All 13C data sets were processed using MATLAB (Mathworks Inc., Natick, MA, USA) as described previously6.Results and Discussion
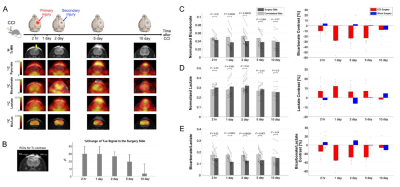
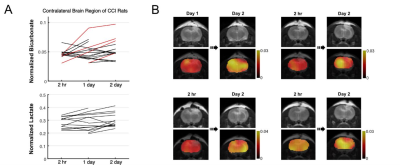
Overall, rats with CCI surgery showed an increase of T2-weighted 1H MRI and hyperpolarized [1-13C]lactate and a decreased of [13C]bicarbonate, Figure 1A. Compared to the contralateral region, T2-hyperintensity peaked rapidly with a contrast of 29.5 ± 10.2 % at 2-hr post-injury (P < 0.01), then slowly decreased at day 1 (P < 0.01), day 2 (P < 0.01), day 5 (P < 0.0001), and day 10 (P = 0.3), Figure 1B. No T2-lesion was evident in the animals with sham surgery. [13C]Bicarbonate production in the T2 hyperintense lesion was slightly lower than the contralateral side at 2-hr post-CCI. The difference in the normalized bicarbonate between the regions peaked at day 1 (P = 0.0006) with a contrast of -28.0 ± 13.2 % in the lesion, started to decrease at day 2 (P = 0.00002), further decreased at day 5 (P = 0.004), and became negligible at day 10 (P = 0.07), Figure 1C. Conversely, lactate production in the lesion was higher at 2-hr post-CCI as compared to the contralateral side (P = 0.02). The greatest increase in [1-13C]lactate production in the lesion relative to the contralateral was detected at day 1 (P = 0.001) and day 2 (P = 0.02). The contrast between the surgery and contralateral sides decreased gradually at day 5 (P = 0.03) and day 10 (P = 0.5), Figure 1D. [13C]Bicarbonate-to-[1-13C]lactate ratio reflected the changes in [13C]bicarbonate and [1-13C]lactate together with enhanced contrast between the lesion and the contralateral up to -35.4 ± 13.8 % (day 1, P = 0.0002), Figure 1E. While the PDH flux in the normal-appearing contralateral brain region was similar to that seen in sham-surgery animals, a subset of the CCI rats showed noticeable increase in bicarbonate production at day 2 post-surgery in the normal-appearing brain, Figure 2A-B. We hypothesize that these results indicate crosstalk between neuronal damage and glial responses to promote allostatic response to ATP levels7, suggesting that the potential compensatory role of undamaged brain tissue while damaged tissue undergoes metabolic transformation. One explanation could be that lactate is being converted back to pyruvate due to the observed apoptosis, which is known to reduce NADH pools8. Another possibility is that the lactate from the lesion is shunted to unimpacted brain tissues for efficient TCA cycle utilization, producing bicarbonate. This short-lived bicarbonate surge was not observed in animals following sham surgery.Conclusion
Understanding the longitudinal changes of cerebral metabolism that occur after TBI is essential for providing adequate patient care. Mitochondrial dysfunction is a major metabolic phenotype of TBI but has been a challenging target to monitor noninvasively in-vivo. This work demonstrates that pyruvate flux into the mitochondria via PDH is sensitive to brain injury and [13C]bicarbonate produced from hyperpolarized [1-13C]pyruvate is a noninvasive imaging biomarker that detects the altered PDH activity in the brain.Acknowledgements
National Institute of Health: R01NS107409, R21EB030765, P30DK127984, P41EB015908 Department of Defense: W81XWH2210485 The Welch Foundation: I-2009-20190330References
1. Selassie, A.W. et al. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil 23, 123-131 (2008).
2. Zaloshnja, E., Miller, T., Langlois, J.A. & Selassie, A.W. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J Head Trauma Rehabil 23, 394-400 (2008).
3. Taylor, C.A., Bell, J.M., Breiding, M.J. & Xu, L. Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013. MMWR Surveill Summ 66, 1-16 (2017).
4. Guglielmetti, C. et al. In vivo metabolic imaging of Traumatic Brain Injury. Sci Rep 7, 17525 (2017).
5. Dixon, C.E., Clifton, G.L., Lighthall, J.W., Yaghmai, A.A. & Hayes, R.L. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods 39, 253-262 (1991).
6. Park, J.M. et al. Measuring mitochondrial metabolism in rat brain in vivo using MR Spectroscopy of hyperpolarized [2-¹³C]pyruvate. NMR Biomed 26, 1197-1203 (2013).
7. Mason S. Lactate Shuttles in Neuroenergetics — Homeostasis, Allostasis and Beyond. Front Neurosci. 2017;11:1–15
8. Miramar MD, Costantini P, Ravagnan L, Saraiva LM, Haouzi D, Brothers G, et al. NADH Oxidase Activity of Mitochondrial Apoptosis-inducing Factor. J Biol Chem. 2001;276(19):16391–8
Figures