0843
Development for hyperpolarized [1-13C]alpha-ketoglutarate MRI for metabolic imaging of normal volunteers and mutant IDH glioma patients1Department of Radiology and Biomedical Imaging, University of California, San Francisco, CA, United States, 2Department of Neurological Surgery, University of California, San Francisco, CA, United States, 3ISOTEC Stable Isotope Division, MilliporeSigma, Miamisburg, OH, United States
Synopsis
Keywords: Hyperpolarized MR (Non-Gas), Non-Proton
Hyperpolarized [1-13C]alpha-ketoglutarate (aKG) is a metabolic probe that can provide direct access to the metabolic pathway for glutamate production or 2-HG production in altered alpha-ketoglutarate metabolism via IDH mutations. With its potential to serve as a biomarker of IDH1 mutational status in glioma and a measure of aKG metabolism in normal healthy subjects, we developed a new chemistry preparation and standard operating procedure (SOP) for on-site production of hyperpolarized GMP [1-13C]alpha-ketoglutarate and characterized the probe to investigate the feasibility of this probe in clinical setting. Following process qualification trials with HP [1-13C]aKG, an FDA IND application was prepared and submitted.Introduction
Hyperpolarized carbon-13 (HP 13C) MRI is an emerging non-radioactive metabolic imaging technology that allows monitoring biochemical reactions in vivo with significantly increased sensitivity via hyperpolarization1. Among the HP 13C probes that have been utilized in preclinical studies, the TCA cycle metabolite [1-13C]alpha-ketoglutarate (aKG) is a promising agent to assess isocitrate dehydrogenase 1 (IDH1) mutational status in glioma and evaluate conversion to glutamate, an important neurotransmitter2,3. The assessment of altered aKG metabolism utilizing HP [13C]aKG has been demonstrated in preclinical IDH-mutant models by detecting the elevated production of oncometabolite 2-hydroxyglutarate and reduced conversion to glutamate4,5. This finding provides a strong rationale to investigate the clinical utility of this novel HP imaging probe to serve as non-invasive markers of altered aKG metabolism in humans as well as a measure of glutamate production. This work developed methods and standard operating procedures (SOP) for routine production of sterile [1-13C]aKG probe for clinical translation of HP [1-13C]aKG.Methods
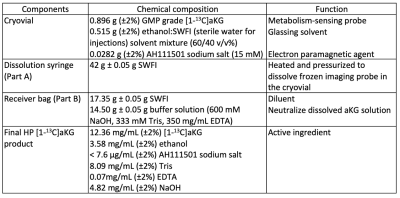
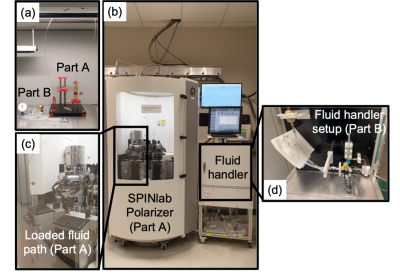
The preparation of HP [1-13C]aKG probe for clinical doses involved dissolving 1.30 g of GMP [1-13C]aKG (ISOTEC) in a mixture of ethanol and H2O (60:40 v/v ratio) and 15 mM of AH111501, electron paramagnetic agent (EPA). 1.44 g of the final solution was loaded into the clinical polarizer (SPINLab polarizer, GE Healthcare) and polarized for 3 hours. The frozen HP aKG was rapidly dissolved with 42 mL of preheated H2O and neutralizing buffer solution. Finally, the dissolved solution was passed through a 0.22 µm sterile filter into a 60 ml Medrad syringe under pharmacist oversight. This process was operated using a GE Fluid Handler (Figure 1). 3 mL of the probe sample was obtained to perform the Quality Control (QC) tests before releasing the injection product for administration. The pre-release QC tests included visual inspection of EPA concentration, pH strip measurement, and sterile filter integrity test. Within a minute after dissolution, >40 mL of a safe, sterile, HP [1-13C]aKG solution was produced at concentrations sufficient for patient imaging (>80 mM). The QC tests performed post-administration included pH electrode measurement, endotoxin and sterility tests.The stability of aKG stock solution was tested using high-field 13C NMR spectroscopy over 2 weeks. The solution was stored at -80°C in aliquots, then thawed and diluted threefold right before the measurements. The recipe for formulating aKG samples to polarize and dissolution media are summarized in Table 1. Three process qualification trials were performed to test the SOP, and toxicology studies were conducted on ratsincluding complete blood counts, liver-kidney function tests, body weight monitoring, and pathological examination at 2 weeks to investigate any potential toxicity of HP [1-13C]aKG and for submission of an IND to the FDA.Results and discussion
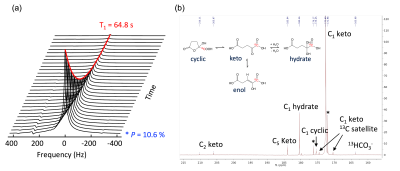
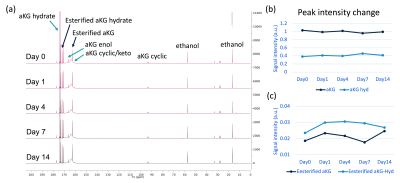
All three PQ trials for HP [1-13C]aKG production were successful, producing ~40 mL of HP [1-13C]aKG at 82.3 ± 3.5 mM and pH 8. Figure 2a shows representative MRS data of HP [1-13C]aKG produced from the PQ. The measured polarization at the first acquired timepoint (72s post-dissolution) was 10.6 % and decayed at a rate of 1/64.8 sec-1 due to T1 relaxation. Based on this, the liquid-state polarization at the time of dissolution can be estimated to be 32 %, comparable to that of HP [1-13C]pyruvate (~ 40%). 13C NMR spectroscopy of the HP [1-13C]aKG probe revealed that it exists in three different forms (i.e. cyclic, keto/enol, and hydrate) under equilibrium as shown in Figure 2b. From the spectrum acquired at thermal equilibrium, it was found that [1-13C]aKG predominantly existed as the keto form with 5~6 % hydrated form and a small amount of cyclic form (< 1%) at pH 8. Additionally, esterification products of [1-13C]aKG and ethanol were present at ~5 % of [1-13C]aKG and observed in the HP spectrum as marked with asterisks in Figure 2b. The esterified aKG such as diethyl aKG has been tested as a cell membrane-permeable precursor of aKG, and no toxicity was reported on this compound6,7. Despite the minor side reactions, the choice for ethanol/water as a solvent mixture was made to help vitrify the aKG solution for efficient hyperpolarization. Also, it facilitated a transfer of the aKG probe during the dissolution due to its low viscosity. Propylene glycol/water and glycerol/water solvent, which are utilized in preclinical studies, led to lower liquid-state aKG probe concentration due to high viscosity. From the aKG stability test with ~ 5.6 M aKG stock solution, the amount of esterification products was consistent as 4.9 ± 0.5 % of keto-aKG over 2 weeks (Figure 3). This result shows that the aKG solution can be made in advance, obviating the need to prepare a fresh solution daily. The sterility tests in all process qualification trials were passed, and no abnormalities of major organs or behavior were observed in the toxicology tests. Based on the developed methods and test results, an FDA IND application for HP [1-13C]aKG was submitted.Conclusion
The study developed methods for producing sterile HP [1-13C]aKG doses to enable metabolic imaging clinical research and successfully completed the process qualification trials. A >30% 13C polarization level achieved on the sterile HP [1-13C]aKG is expected to provide a new metabolic probe for assessing aKG metabolism and glutamate production in humans.Acknowledgements
This research was supported by the NIH (P01CA118816, P41EB013598) and the UCSF NICO project.References
1. Chaumeil, M.M., C. Najac, and S.M. Ronen, Studies of Metabolism Using (13)C MRS of Hyperpolarized Probes. Methods Enzymol, 2015. 561: p. 1-71.
2. Chaumeil, M.M., et al., Hyperpolarized [1-13C] glutamate: a metabolic imaging biomarker of IDH1 mutational status in glioma. Cancer Res, 2014. 74(16): p. 4247-57.
3. Chaumeil, M.M., et al., Non-invasive in vivo assessment of IDH1 mutational status in glioma. Nat Commun, 2013. 4: p. 2429.
4. Hong, D., et al., Acquisition and quantification pipeline for in vivo hyperpolarized 13C MR spectroscopy. Magn Reson Med, 2022. 87(4): p. 1673-1687.
5. Hong, D., et al., Direct detection of 2HG and glutamate production using hyperpolarized [1-13C-5-12C]-a-ketoglutarate in cell and in vivo glioma models. ISMRM 2022, London. Program Number: 1064
6. AbuSalim, J.E., et al., Simple Esterification of [1-13C]-Alpha-Ketoglutarate Enhances Membrane Permeability and Allows for Non-invasive Tracing of Glutamate and Glutamine Production. ACS Chem Biol. 2021, 16: p. 2144-2150.
7. Singh, J., 13C‐Labeled Diethyl Ketoglutarate Derivatives as Hyperpolarized Probes of 2‐Ketoglutarate Dehydrogenase Activity. Analysis & Sensing 2021, 1(4): p. 156-160
Figures