0841
Hyperpolarized δ-[1-13C]-gluconolactone reports on TERT expression and response to therapy in brain tumors1University of California, San Francisco, San Francisco, CA, United States
Synopsis
Keywords: Hyperpolarized MR (Non-Gas), Cancer, animals, preclinical, brain, metabolism
TERT expression is essential for telomere maintenance and uncontrolled tumor proliferation in oligodendrogliomas. TERT is an attractive therapeutic target and the drug 6-thio-2’-deoxyguanosine that disrupts telomere maintenance is in clinical trials for cancer. We previously showed that TERT expression is associated with upregulation of the pentose phosphate pathway in oligodendrogliomas. Here, we have established the ability of hyperpolarized δ-[1-13C]-gluconolactone, a probe of the pentose phosphate pathway, to assess TERT expression and response to 6-thio-2’-deoxyguanosine in oligodendrogliomas in vivo. Our findings provide a non-invasive method of imaging a hallmark of cancer and have the potential to improve management of oligodendroglioma patients.INTRODUCTION
Telomere maintenance by telomerase reverse transcriptase (TERT) is essential for immortality in cancer, including oligodendrogliomas1,2. Due to this key role, agents that disrupt telomere maintenance such as 6-thio-2’-deoxyguanosine (6-thio-dG) are in clinical trials3-6. We previously showed that TERT expression in oligodendrogliomas is associated with upregulation of glucose-6-phosphate dehydrogenase (G6PD), the rate-limiting enzyme of the pentose phosphate pathway (PPP)7. We also showed that hyperpolarized δ-[1-13C]-gluconolactone metabolism to 6-phosphogluconate (6-PG) can probe the PPP in glioblastomas8. The goal of this study was to determine whether hyperpolarized δ-[1-13C]-gluconolactone monitors TERT expression and response to 6-thio-dG in oligodendrogliomas.METHODS
Cell models: We examined patient-derived oligodendroglioma models (BT88, BT54, SF10417)7,9. TERT expression was silenced using siRNAs against TERT or non-targeting control siRNA7,9.δ-[1-13C]-gluconolactone preparation: δ-[1-13C]-gluconolactone was synthesized and polarized as previously described8. 2M δ-[1-13C]-gluconolactone was dissolved in 3:1 water:glycerol and mixed with 15mM trityl radical OX063. After maximal polarization was achieved, samples were dissolved in 6ml (cell studies) or 3.9ml (in vivo) of phosphate-buffered saline (pH 7).
Hyperpolarized 13C-MRS in live cells: Hyperpolarized δ-[1-13C]-gluconolactone was injected into a suspension of live cells (~3x107) in a 5mm NMR tube. 13C spectra were acquired on a 11.7T Varian spectrometer with a 13° flip angle every 3s for 300s. Data was analyzed using Mnova. δ-gluconolactone is in equilibrium with γ-gluconolactone in aqueous solutions and we previously confirmed that differences in the relative levels of δ- and γ-gluconolactone did not influence 6-PG production8. Therefore, we evaluated the ratio of [1-13C]-6-PG to the combined signal from δ-[1-13C]-gluconolactone and γ-[1-13C]-gluconolactone (henceforth referred to as total [1-13C]-gluconolactone) and to cell number.
MRI: Glioma cells were intracranially injected into athymic male nude rats7-9. Tumor volume was calculated by T2-weighted MRI performed on a Bruker 3T scanner equipped with a dual-tuned 1H-13C volume coil using a spin-echo TurboRARE sequence7-9. For assessment of response to 6-thio-dG, once tumors reached a volume of 64.8±36.1mm3, this timepoint was considered day 0. Rats were then treated intraperitoneally with 6-thio-dG (50mg/kg in saline) daily for 7 days and then 4 times a week.
Hyperpolarized 13C-MRS in vivo: 2.2ml of hyperpolarized δ-[1-13C]-gluconolactone (final concentration 37.8mM) was intravenously injected. For non-localized slab studies, dynamic 13C spectra were acquired from a 12mm axial slab through the brain every 3s using a flyback spectral-spatial RF pulse with flip angles of 15.3° on [1-13C]-6-PG, 3.4o on δ-[1-13C]-gluconolactone and 12o on γ-[1-13C]-gluconolactone. 13C slab spectra were analyzed using Mnova and metabolite SNR quantified. For spatial localization, data was acquired using a 2D flyback spectral-spatial echo-planar spectroscopic imaging (EPSI) pulse with flip angles as described above8. The spatial resolution of the EPSI method was 5.375x5.375x8mm3, the temporal resolution was 3s, and the spectral resolution was 128 points over 20ppm. 2D EPSI data was analyzed using in-house MATLAB codes7-9.
Statistical analysis: Results are expressed as mean±STD. Statistical significance was assessed using an unpaired two-tailed Welch’s t-test with p<0.05 considered significant.
RESULTS
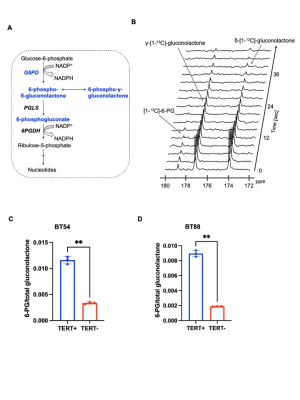
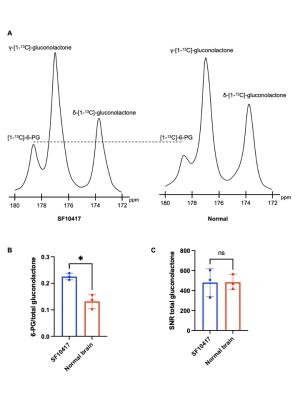
Hyperpolarized δ-[1-13C]-gluconolactone reports on TERT expression in patient-derived oligodendroglioma cells: Our prior studies indicate that silencing TERT down-regulates G6PD and reduces glucose flux via the PPP in oligodendroglioma cells7. Since hyperpolarized δ-[1-13C]-gluconolactone is metabolized via the PPP downstream of G6PD8,10 (Figure 1A), we examined whether hyperpolarized δ-[1-13C]-gluconolactone provides a readout of TERT expression in oligodendroglioma cells. As shown in Figure 1B-1D, silencing TERT significantly reduced 6-PG production from hyperpolarized δ-[1-13C]-gluconolactone in both BT88 and BT54 models.Hyperpolarized δ-[1-13C]-gluconolactone reports on TERT expression in patient-derived oligodendrogliomas in vivo: Next, we examined the feasibility of imaging TERT in vivo. Dynamic 13C spectra were acquired from a slab through the brain of SF10417 tumor-bearing rats or tumor-free controls following intravenous injection of hyperpolarized δ-[1-13C]-gluconolactone. As shown in Figure 2A-2B, the [1-13C]-6-PG/total [1-13C]-gluconolactone was significantly higher in rats bearing TERT+ SF10417 tumors relative to TERT- normal brain. In contrast, there was no difference in the SNR of total [1-13C]-gluconolactone (Figure 2C), indicating that the differences in 6-PG production were not the result of differences in gluconolactone delivery.
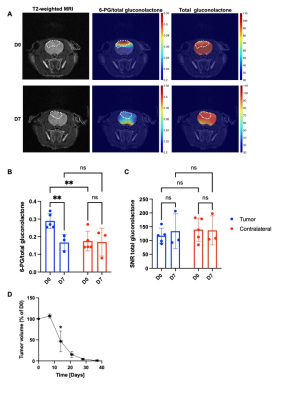
Hyperpolarized δ-[1-13C]-gluconolactone reports on early response to therapy in vivo: 6-thio-dG induces cell death in TERT+ cancer cells and tumors3-6. We treated rats bearing orthotopic BT88 tumors with 6-thio-dG and examined hyperpolarized δ-[1-13C]-gluconolactone metabolism before (day 0) and after (day 7) treatment. 6-PG production from hyperpolarized δ-[1-13C]-gluconolactone was significantly reduced at day 7 relative to day 0 specifically in the tumor region but not in contralateral normal brain (Figure 3A-3B). In contrast, there was no significant difference in the SNR of total [1-13C]-gluconolactone in either tumor or in contralateral normal brain at day 7 vs. day 0 (Figure 3A, 3C). Importantly, reduced tumor volume only at day 14 (Figure 3D), indicating that the reduction in 6-PG production occurs before tumor shrinkage. Collectively, our results indicate that hyperpolarized δ-[1-13C]-gluconolactone provides a readout of response to 6-thio-dG at early time points before MRI-detectable anatomical alterations in vivo.
CONCLUSIONS
Our findings indicate that 6-PG production from hyperpolarized δ-[1-13C]-gluconolactone provides a readout of TERT expression and early response to therapy in rats bearing orthotopic patient-derived oligodendrogliomas in vivo. Clinical translation of hyperpolarized δ-[1-13C]-gluconolactone has the potential to provide a readout of tumor burden and response to therapy in oligodendroglioma patients.Acknowledgements
We would like to thank Joseph F Costello for providing the SF10417 model. We thank Dr. Luchman and Dr. Cairncross for providing us the BT54 and BT88 models. We thank Will Byrne for his technical support in the Preclinical MR Imaging laboratory. These studies were funded by the Department of Defense (W81XWH201055315), National Institutes of Health (R01CA239288) and the UCSF NICO initiative. The authors also acknowledge technical support from the National Institutes of Health-supported Hyperpolarized MRI Technology Resource Center (P41EB013598).References
1. Shay JW, Wright WE. Telomeres and telomerase: three decades of progress. Nature Reviews Genetics. 2019; 20(5):299-309.
2. Bell RJ, Rube HT, Xavier-Magalhaes A, et al. Understanding TERT Promoter Mutations: A Common Path to Immortality. Molecular cancer research : MCR. 2016; 14(4):315-323.
3. Mender I, Gryaznov S, Dikmen ZG, Wright WE, Shay JW. Induction of telomere dysfunction mediated by the telomerase substrate precursor 6-thio-2'-deoxyguanosine. Cancer discovery. 2015; 5(1):82-95.
4. Sengupta S, Sobo M, Lee K, et al. Induced Telomere Damage to Treat Telomerase Expressing Therapy-Resistant Pediatric Brain Tumors. Molecular cancer therapeutics. 2018; 17(7):1504-1514.
5. Mender I, Gryaznov S, Shay JW. A novel telomerase substrate precursor rapidly induces telomere dysfunction in telomerase positive cancer cells but not telomerase silent normal cells. Oncoscience. 2015; 2(8):693-695.
6. Mender I, LaRanger R, Luitel K, et al. Telomerase-Mediated Strategy for Overcoming Non-Small Cell Lung Cancer Targeted Therapy and Chemotherapy Resistance. Neoplasia (New York, N.Y.). 2018; 20(8):826-837.
7. Viswanath P, Batsios G, Ayyappan V, et al. Metabolic imaging detects elevated glucose flux through the pentose phosphate pathway associated with TERT expression in low-grade gliomas. Neuro-oncology. 2021.
8. Batsios G, Taglang C, Cao P, et al. Imaging 6-Phosphogluconolactonase Activity in Brain Tumors In Vivo Using Hyperpolarized δ-[1-(13)C]gluconolactone. Frontiers in oncology. 2021; 11:589570.
9. Viswanath P, Batsios G, Mukherjee J, et al. Non-invasive assessment of telomere maintenance mechanisms in brain tumors. Nature communications. 2021; 12(1):92.
10. Moreno KX, Harrison CE, Merritt ME, Kovacs Z, Malloy CR, Sherry AD. Hyperpolarized delta-[1-(13) C]gluconolactone as a probe of the pentose phosphate pathway. NMR in biomedicine. 2017; 30(6).
Figures