0839
Pre-treatment hyperpolarized 13C-lactate to 13C-bicarbonate ratio predicts response of brain metastases to stereotactic radiosurgery1Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 2Radiation Oncology, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 3Physical Sciences, Sunnybrook Research Institute, Toronto, ON, Canada, 4GE Healthcare, Toronto, ON, Canada, 5Pharmacy, Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 6Radiology, Sunnybrook Health Sciences Centre, Toronto, ON, Canada
Synopsis
Keywords: Hyperpolarized MR (Non-Gas), Cancer, Treatment Response Prediction, Radiotherapy, Brain Metastases, Metabolism
Brain metastases are increasingly being treated with stereotactic radiosurgery; however, 20-30% of treated tumors locally recure post treatment. Hyperpolarized [1-13C]pyruvate magnetic resonance imaging (HP 13C MRI) is an emerging metabolic imaging modality that measures key metabolic phenotypes indicative of aggressive tumor phenotypes. Here we show that the pre-treatment tumor 13C-lactate to 13C-bicarbonate ratio – a marker of glycolysis and (indirectly) oxidative phosphorylation – measured via HP [1-13C]pyruvate MRI is a robust predictor of local recurrence (AUCROC=0.95, p=0.0008; AUCPRC=0.92) and can inform treatment decisions should the model predict a non-response to SRS.Introduction
Brain metastases (BMs) complicate upwards of 40% of cancer patients1–4 and are commonly treated with stereotactic radiosurgery (SRS), surgery, or a combination of the two1,5. Although SRS is an effective treatment compared to whole brain radiotherapy, still 20-30% of BMs recur locally6. In such cases, an alternative radiation schedule or surgery may have been superior; however, current response assessment methods rely on detecting changes in tumor volume, which take 4-6 weeks to stabilize7. Functional imaging modalities, on the other hand, have the potential to assess treatment response pre-treatment or shortly following therapy initiation.Hyperpolarized [1-13C]pyruvate magnetic resonance imaging (HP 13C MRI) is an emerging metabolic imaging modality that allows for a 104-fold increase in the signal-to-noise ratio of key metabolites in vivo8,9 and the interrogation of key metabolic reactions that 18F-flurodeoxyglucose positron emission tomography cannot. The majority of HP 13C MRI oncology studies have focused on pyruvate-to-lactate conversion to measure glycolysis within tumors. Pyruvate, however, lies at a critical branching point where it can either be converted to lactate or acetyl-CoA and CO2 (producing bicarbonate – a surrogate for oxidative phosphorylation in the brain, where conversion of acetyl-CoA to non-tricarboxylic acid pathways is low)10,11. Thus, the lactate-to-bicarbonate ratio provides an indirect means of measuring the proportion of glycolysis to oxidative phosphorylation. Using the lactate-to-bicarbonate ratio is recommended over the lactate-to-pyruvate ratio when evaluating treatment response to therapies inducing hypoxia12 – as in the case with SRS where severe vascular damage occurs13. Two pre-clinical glioma studies used HP 13C MRI to predict early anti-VEGF treatment response and found the lactate-to-bicarbonate ratio predictive of response 48h post treatment and correlated with survival11,14. Therefore, we hypothesize that a high pre-treatment tumor lactate-to-bicarbonate ratio measured in vivo with HP 13C MRI can predict non-responders to SRS.
Methods
Written informed consent was obtained from N=12 patients with BMs (M=18 tumors) under a protocol approved by the Sunnybrook Research Ethics Board and Health Canada. A 0.43 mL/kg dose of 250 mM [1-13C]pyruvate was prepared in a sterile fluid path and hyperpolarized in a GE SPINLab polarizer. Participants were scanned using a GE MR750 3.0T MRI scanner (GE Healthcare, WI) with their head secured in the support of a standard 8-channel neurovascular receive array (Invivo Inc.). [1-13C]pyruvate was intravenously injected at 4 mL/s, followed by a 25 mL saline flush at 5 ml/s. A custom 13C birdcage coil and 3D dual-echo echo-planar imaging sequence was used to acquire time-resolved volumetric images of pyruvate, lactate and bicarbonate (5s temporal resolution; 1.5cm isotropic spatial resolution; 24×24×36cm3 field of view)15. Following 13C image acquisition, the 8-channel 1H neurovascular array was used to acquire 1H T1-w, gadolinium (Gd) enhanced T1-w, and T2-FLAIR images.13C image reconstruction was done in MATLAB (MathWorks Inc., MA). Time-resolved 13C images were summed to compute the area under the curve (AUC) for pyruvate, lactate and bicarbonate. Tumors were contoured by a radiation oncologist on T1-w or T2-w images if Gd enhanced T1-w images were not available. Mean metabolite signal was calculated for each tumor. Tumor response was determined according to the RANO-BM criteria7. The predictive power of 13C metabolite signals were compared to literature-derived Graded Prognostic Assessment16 and clinical parameters, such as the Karnofsky performance status (KPS), age, number of metastatic sites, number of central nervous system (CNS) metastases, radiation dose, and tumor volume.
Results & Discussion
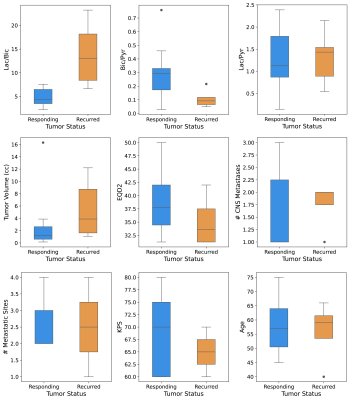
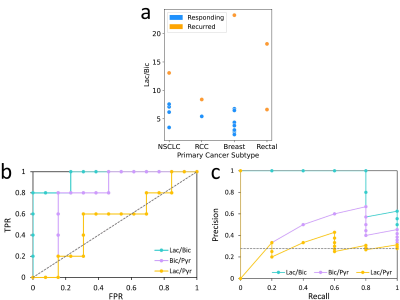
Figure 1 demonstrates the differences in the three metabolite ratios and six clinical parameters, between responding and non-responding tumors. The lactate-to-bicarbonate (p=0.0008) and bicarbonate-to-pyruvate (p=0.03) ratios were significantly different between the two groups (one-sided Mann Whitney U Test, α=0.05). No other parameter was able to distinguish the two groups. Although tumor volume approached statistical significance, it is known that larger tumors do not respond well to single SRS doses; tumors are sent for surgical resection or fractionated SRS.To assess the predictive power of the top performing metabolite ratios, receiver operating characteristic curve (ROC) and precision recall curve (PRC) analysis was conducted. The lactate-to-bicarbonate ratio established a robust predictive model, returning an AUCROC=0.95 (p=0.0008) and optimal threshold resulting in a true positive rate of 0.8 and false positive rate of 0 (Figure 2). The statistical significance of this result was evaluated using the Mann-Whitney U Test17, with α=0.05. The AUCPRC was also evaluated due to class imbalance; the AUCPRC was still encouraging at 0.92 for lactate-to-bicarbonate. For the bicarbonate-to-pyruvate ratio, the AUCROC was 0.78; the AUCPRC gave a better estimate of the classifier’s accuracy, however, it was not predictive (AUCPRC=0.43). Similarly, the lactate-to-pyruvate ratio was not predictive.
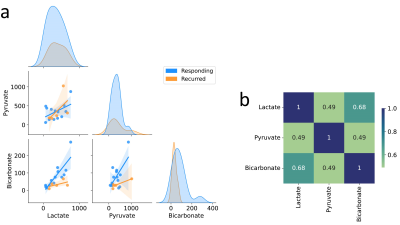
Finally, combinations of lactate, bicarbonate and pyruvate were assessed for their potential to differentiate responders from non-responders. Preliminary analysis revealed high correlations between the three metabolites (Figure 3), suggesting that using all metabolites in a predictive classifier model may be redundant. Predictions based off single metabolites have been explored by our group18,19, however, the lactate-to-bicarbonate ratio outperforms these models. Overall, the lactate-to-bicarbonate ratio outperforms select clinical parameters, single metabolites models, and current radiomics based models20.
Conclusions
The lactate-to-bicarbonate ratio of brain metastases is a robust predictive marker of local recurrence and could inform treatment decisions for tumors unlikely to respond to SRS.Acknowledgements
We would like to acknowledge the following sources of funding: The Canadian Cancer Society Research Institute, NSERC: RGPIN-2016-05566, and CIHR: CIHR PJT152928.References
1. Soliman H, Das S, Larson DA, Sahgal A. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget. 2016;7(11):12318-12330. doi:10.18632/oncotarget.7131
2. Nayak L, Lee EQ, Wen PY. Epidemiology of Brain Metastases. Curr Oncol Rep. 2012;14(1):48-54. doi:10.1007/s11912-011-0203-y
3. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence Proportions of Brain Metastases in Patients Diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-2872. doi:10.1200/JCO.2004.12.149
4. Tsukada Y, Fouad A, Pickren JW, Lane WW. Central nervous system metastasis from breast carcinoma autopsy study. Cancer. 1983;52(12):2349-2354. doi:https://doi.org/10.1002/1097-0142(19831215)52:12<2349::AID-CNCR2820521231>3.0.CO;2-B
5. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225. doi:10.1016/j.prro.2011.12.004
6. Chao ST, De Salles A, Hayashi M, et al. Stereotactic Radiosurgery in the Management of Limited (1-4) Brain Metasteses: Systematic Review and International Stereotactic Radiosurgery Society Practice Guideline. Neurosurgery. 2018;83(3):345-353. doi:10.1093/neuros/nyx522
7. Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270-e278. doi:10.1016/S1470-2045(15)70057-4
8. Wolber J, Ellner F, Fridlund B, et al. Generating highly polarized nuclear spins in solution using dynamic nuclear polarization. Nucl Instrum Methods Phys Res Sect Accel Spectrometers Detect Assoc Equip. 2004;526(1-2):173-181. doi:10.1016/j.nima.2004.03.171
9. Ardenkjaer-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci. 2003;100(18):10158-10163. doi:10.1073/pnas.1733835100
10. Park JM, Josan S, Grafendorfer T, et al. Measuring mitochondrial metabolism in rat brain in vivo using MR Spectroscopy of hyperpolarized [2-13C]pyruvate. NMR Biomed. 2013;26(10):1197-1203. doi:10.1002/nbm.2935
11. Park JM, Spielman DM, Josan S, et al. Hyperpolarized 13C-lactate to 13C-bicarbonate ratio as a biomarker for monitoring the acute response of anti-vascular endothelial growth factor (anti-VEGF) treatment. NMR Biomed. 2016;29(5):650-659. doi:10.1002/nbm.3509
12. Julià-Sapé M, Candiota AP, Arús C. Cancer metabolism in a snapshot: MRS(I). NMR Biomed. 2019;32(10):e4054. doi:10.1002/nbm.4054
13. Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-Induced Vascular Damage in Tumors: Implications of Vascular Damage in Ablative Hypofractionated Radiotherapy (SBRT and SRS). Radiat Res. 2012;177(3):311-327. doi:10.1667/RR2773.1
14. Datta K, Lauritzen MH, Merchant M, et al. Reversed metabolic reprogramming as a measure of cancer treatment efficacy in rat C6 glioma model. Dadachova E, ed. PLOS ONE. 2019;14(12):e0225313. doi:10.1371/journal.pone.0225313
15. Geraghty BJ, Lau JYC, Chen AP, Cunningham CH. Dual-Echo EPI sequence for integrated distortion correction in 3D time-resolved hyperpolarized 13C MRI. Magn Reson Med. 2018;79(2):643-653. doi:10.1002/mrm.26698
16. Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A New Prognostic Index and Comparison to Three Other Indices for Patients With Brain Metastases: An Analysis of 1,960 Patients in the RTOG Database. Int J Radiat Oncol Biol Phys. 2008;70(2):510-514. doi:10.1016/j.ijrobp.2007.06.074
17. Grunkemeier GL, Jin R. Receiver operating characteristic curve analysis of clinical risk models. Ann Thorac Surg. 2001;72(2):323-326. doi:10.1016/S0003-4975(01)02870-3
18. Lee CY, Soliman H, Bragagnolo ND, et al. Predicting response to radiotherapy of intracranial metastases with hyperpolarized 13C MRI. J Neurooncol. Published online March 19, 2021. doi:10.1007/s11060-021-03725-7
19. Cappelletto NIC, Soliman H, Lee CY, et al. Evaluating hyperpolarized [1-13C]pyruvate uptake for predicting response to stereotactic radiosurgery in brain metastases. Paper presented at: 31st Annual Meeting of the International Society for Magnetic Resonance in Medicine; May 11, 2022; London, UK.
20. Mouraviev A, Detsky J, Sahgal A, et al. Use of radiomics for the prediction of local control of brain metastases after stereotactic radiosurgery. Neuro-Oncol. 2020;22(6):797-805. doi:10.1093/neuonc/noaa007
Figures