0807
Towards a probabilistic structural atlas of brainstem nuclei in elderly humans using in-vivo 7 Tesla multi-contrast MRI1Brainstem Imaging Laboratory, Department of Radiology, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States, 2Escuela Nacional de Estudios Superiores Unidad Juriquilla, Universidad Nacional Autónoma de México, Queretaro, Mexico, 3Division of Sleep Medicine, Harvard University, Boston, MA, United States
Synopsis
Keywords: Segmentation, Aging, Brainstem atlas
Brainstem nuclei are involved in several vital functions; impairment in their structure and function is manifested in several clinical conditions of elderly humans, including sleep/arousal/movement/vestibular/anxiety disorders, chronic pain and altered autonomic functions. Brainstem evaluation in health and disease is currently limited by the difficulty of localizing these regions in conventional MRI. We developed an in-vivo probabilistic atlas of 31 brainstem nuclei in elderly humans by the use of multi-contrast 7 Tesla MRI in 15 elderly subjects and of an existing brainstem nuclei atlas in younger adults. This atlas can be applied to conventional MRI and aid brainstem investigation in aging.
Introduction:
Brainstem nuclei are involved in several vital functions, such as sleep, arousal, motor, sensory, limbic, nociceptive and autonomic function1. Changes in brainstem nuclei structure and function due to aging, disease or injury are manifested in several clinical conditions of elderly humans, including sleep/movement/vestibular/anxiety disorders, as well as chronic pain and altered autonomic functions2-7. Nevertheless, evaluation of structural and functional alterations of these nuclei is difficult in research and clinical studies, because of the limited image spatial resolution and contrast of conventional (e.g. 3 Tesla) MRI scanners and the lack of a probabilistic brainstem nuclei atlas of living elderly humans.Purpose:
To develop a probabilistic structural atlas of 31 brainstem nuclei and an optimal multi-contrast template image of the brainstem in living elderly humans, by the use of high-resolution (1.1 mm3 isotropic) multi-contrast (diffusion fractional anisotropy (FA) and T2-weighted) EPI approach at 7 Tesla and of an existing brainstem nuclei atlas (Brainstem Navigator) in younger adults8-13.Method:
Data acquisition: Twenty-four subjects (12m/12f, mean ± s.e. age 65 ± 1) were scanned with 7 Tesla MRI under IRB approval. A common single-shot 2D EPI readout was adopted for 1.1 mm3 isotropic diffusion-tensor (DTI), and T2-weighted (T2w) sagittal images, with matrix size/GRAPPA factor/nominal echo-spacing = 180 × 240/3/0.82 ms. This yielded multi-contrast anatomical images with exactly matched resolution and geometric distortions. Additional parameters for the diffusion acquisition were: spin-echo EPI, 61 slices, TE/TR = 60.8 ms/5.6 s, partial Fourier: 6/8, unipolar diffusion-weighting gradients for DTI, 60 diffusion directions (b-value ~ 1000 s/mm2), 7 interspersed “b0” images (non-diffusion weighted, b-value ~ 0 s/mm2), 4 repetitions, acquisition time/repetition 6’43”. Data analysis: Creation of an optimal bivariate FA/T2w brainstem template of elderly humans: First, we rotated DTIs to standard orientation, followed by denoising (local PCA14), motion and distortion correction (FSL), and bias field correction (SPM). Then the diffusion tensor, fractional anisotropy (FA), and S0 image (i.e., T2w MRI) were computed from the preprocessed DTI (dtifit, FSL). We excluded nine subjects because of poor coverage/sensitivity in the medulla and lower pons. Then, the template was created using an iterative approach, which included automatic approximation of individual subject image mask from a preliminary optimal template mask. Specifically, the FA/T2w data of the remaining 15 subjects (8m/7f) was used to create a preliminary (1st iteration) bivariate optimal template (FA and T2w) by iterative averaging and registration (ANTs)15 (transformation model/max iterations/similarity metric/shrink factors/smoothing kernels = greedy symmetric normalization/30×20×4/cross-correlation/4×2×1/2×1×0). The T2w optimal template image was masked (BET, FSL), and inverse transformed to the native space of each T2w image of 15 subjects to create an automatic approximated individual image mask. Next, the masked single-subject images (FA and T2w) were employed to create an improved and finalized (2nd iteration) bivariate optimal template of the brainstem in elderly subjects (ANTs). Brainstem nuclei atlas generation in elderly humans: First, we computed the transformation of the optimal FA/T2w template of elderly subjects to the IIT FA/T2w template of younger adults (transformation model/similarity metric/shrink factors/smoothing kernels = greedy symmetric normalization/cross-correlation/6×4×2×1/3×2×1×0), which is also the space of the Brainstem Navigator atlas in younger adults (https://www.nitrc.org/projects/brainstemnavig/). Subsequently, the single-subject atlas labels of 31 brainstem nuclei (total of 76 labels considering left/right/medial nuclei and subnuclei) of 12 young adults8-13 were brought to the optimal template space of elderly subjects using an inverse non-linear transformation (ANTs); the probabilistic atlas of 76 labels was finally developed by computing the label spatial overlap across subjects. Atlas validation: We validated the probabilistic nuclei atlas in elderly humans by computing the internal consistency across subjects of each label, as the modified Hausdorff distance16 between each label and the probabilistic atlas label (thresholded at 35%) generated by averaging the labels across the other 11 subjects (leave-one-out cross validation). For each nucleus, the across-subject mean/s.e. of the Hausdorff distance was evaluated and displayed.Results:
In Figure 1, we show morphological changes in the brainstem due to aging. In Figures 2-3, we display the probabilistic structural labels of 31 brainstem nuclei (right or medial nuclei displayed) in the optimal template space of elderly subjects. For each nucleus atlas label, the average modified Hausdorff distance evaluating the label internal consistency within the group (Figure 4) was always below (p < 0.05, unpaired t-test) the linear spatial imaging resolution (1.1 mm), thus validating the generated probabilistic brainstem nuclei atlas.Discussion and Conclusions:
This work exhibits the feasibility of creating a preliminary atlas of brainstem nuclei in elderly humans by applying high dimensional non-linear transformations to a current atlas of brainstem nuclei in younger adults. These transformations accounted for larger morphological deformations due to aging, such as enlarged ventricular spaces (Figure 1). These atlas labels can be used as prior for supervised automatic segmentations of these nuclei in multi-contrast images of elderly subjects, which might further improve the delineation of these nuclei in the elderly population. This atlas and template can be applied to conventional MRI of elderly subjects and aid the evaluation of lesions and the assessment of connectivity pathways underlying sleep, arousal, motor, sensory, limbic, nociceptive, and autonomic mechanisms in a broad set of clinical conditions relating to these nuclei.Acknowledgements
NIH NIA R01 AG063982; Dr. Thorsten Feiweier for providing the diffusion sequence used in this study.
References
1. Olszewski J and Baxter D. Cytoarchitecture of the human brain stem. Basel: Karger; 1954.
2. Postuma R B, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019;142(3):744-759.
3. Balaban C D, Jacob R G, Furman J M. Neurologic bases for comorbidity of balance disorders, anxiety disorders and migraine: neurotherapeutic implications. Expert Rev Neurother. 2011;11(3):379-394.
4. Hoffmeister J D, Kelm-Nelson C A, Ciucci M R. Quantification of brainstem norepinephrine relative to vocal impairment and anxiety in the Pink1-/-rat model of Parkinson disease. Behav Brain Res. 2021;414:113514.
5. Braak H, Ghebremedhin E, Rüb U, et al. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318(1):121-134.
6. Raver C, Uddin O, Ji Y, et al. An amygdalo-parabrachial pathway regulates pain perception and chronic pain. J Neurosci. 2020;40(17):3424-3442.
7. Balaban C D. Projections from the parabrachial nucleus to the vestibular nuclei: potential substrates for autonomic and limbic influences on vestibular responses. Brain Res. 2004;996(1):126-137.
8. Bianciardi M, Toschi N, Edlow B L, et al. Toward an in vivo neuroimaging template of human brainstem nuclei of the ascending arousal, autonomic, and motor systems. Brain Connect. 2015;5(10):597-607.
9. Bianciardi M, Strong C, Toschi N, et al. A probabilistic template of human mesopontine tegmental nuclei from in vivo 7 T MRI. Neuroimage. 2018;170:222-230.
10. García-Gomar M G, Strong C, Toschi N, et al. In vivo probabilistic structural atlas of the inferior and superior colliculi, medial and lateral geniculate nuclei and superior olivary complex in humans based on 7 tesla MRI. Front Neurosci. 2019;13:764.
11. Singh K, Indovina I, Augustinack J C, et al. Probabilistic template of the lateral parabrachial nucleus, medial parabrachial nucleus, vestibular nuclei complex, and medullary viscero-sensory-motor nuclei complex in living humans from 7 Tesla MRI. Front Neurosci. 2020;13:1425.
12. Singh K, García-Gomar M G, Bianciardi M. Probabilistic atlas of the mesencephalic reticular formation, isthmic reticular formation, microcellular tegmental nucleus, ventral tegmental area nucleus complex, and caudal–rostral linear raphe nucleus complex in living humans from 7 Tesla magnetic resonance imaging. Brain Connect. 2021;11(8):613-623.
13. García‐Gomar M G, Videnovic A, Singh K, et al. Disruption of brainstem structural connectivity in REM sleep behavior disorder using 7 Tesla magnetic resonance imaging. Mov Disord. 2022;37(4):847-853.
14. Manjón J V, Coupé P, Concha L, et al. Diffusion weighted image denoising using overcomplete local PCA. PLoS One. 2013;8(9):e73021.
15. Avants B B, Tustison N J, Song G, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033-2044.
16. Dubuisson M P, Jain A K. A modified Hausdorff distance for object matching. Proc. 12th International Conference on Pattern Recognition, Jerusalem, Israel, 1, 566-568; 1994.
17. Keuken M C, Bazin P L, Schäfer A, et al. Ultra-high 7T MRI of structural age-related changes of the subthalamic nucleus. J Neurosci. 2013;33(11):4896-4900.Figures
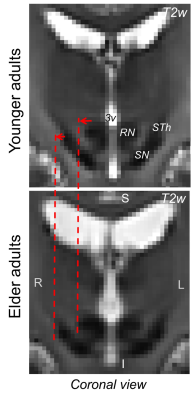
Figure 1 Structural brainstem changes in aging. We show an optimal brainstem template achieved in (top) younger8 and (bottom) elderly adults. Aging entails changes in brain morphology and tissue properties, including enlargement of ventricular spaces and iron accumulation in some brain areas17. As visible here, this is also the case for ventricular areas neighboring the brainstem, such as the third ventricle (3v). Note the associated shift of brainstem nuclei (such as the substantia nigra, SN, and red nucleus, RN) in the lateral direction by 1-3 mm (red arrows).
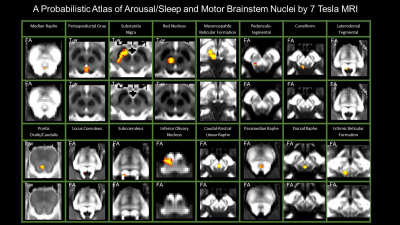
Figure 2 Probabilistic atlas labels of 16 arousal/sleep and motor brainstem nuclei in the optimal template space of elderly subjects (n = 15). Good spatial agreement was achieved for all labels across subjects in the optimal template space of elderly subjects (spatial probabilistic overlap between 35 % and 100 % displayed in red-yellow).
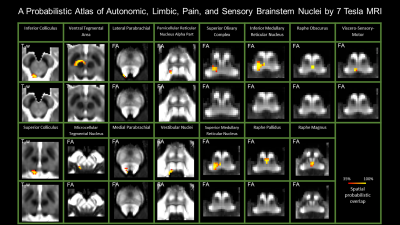
Figure 3 Probabilistic atlas labels of 15 autonomic, limbic, pain and sensory brainstem nuclei in optimal template space of elderly subjects (n = 15). Good spatial agreement was achieved for all labels across subjects in the optimal template space of elderly subjects.
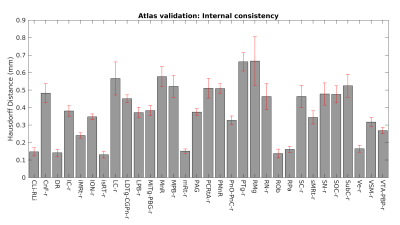
Figure 4 Atlas validation. The internal consistency of 31 brainstem nuclei (right or medial) labels across subjects in the optimal template space of elderly subjects (bar/errorbar plot of mean/standard error of the modified Hausdorff distance) was always lower (p < 0.05) than the spatial imaging resolution, indicating the efficiency of this probabilistic atlas.