0795
Subclassification of Barcelona Clinic Liver Cancer Stage A HCC using fully automatic 3D segmentation-derived total tumor burden at MRI1West China Hospital, Sichuan University, Chengdu, China, 2Shukun (Beijing) Technology Co., Ltd, Beijing, China
Synopsis
Keywords: Liver, Cancer, Carcinoma, hepatocellular; Barcelona Clinic Liver Cancer; Overall survival; Tumor burden
In the present study of 297 patients, we evaluated the role of three-dimensional (3D) quantitative tumor burden analysis using fully automatic segmentation at magnetic resonance imaging in the subcategorization of the Barcelona Clinic Liver Cancer (BCLC) stage A hepatocellular carcinoma (HCC) after curative resection. Our results demonstrated that 3D quantitative total tumor burden (TTB) and serum a-fetoprotein were independent predictors of overall survival and could be used to subcategorize the BCLC stage A HCC. Additionally, the higher TTB (>18.5%) was correlated to more aggressive tumor behaviors (i.e., microvascular invasion and poor tumor differentiation).Abstract
INTRODUCTION: Tumor burden conveys important prognostic implications and impacts the management decisions. Barcelona Clinic Liver Cancer (BCLC) stage A hepatocellular carcinoma (HCC), defined as solitary tumor irrespective of size or as a multifocal tumor up to 3 nodules (none of them >3 cm), consists of highly heterogeneous tumors with various extents of tumor burden1. However, the current BCLC algorithm has relied on one-dimensional measurements of the maximum tumor diameter and number of tumors, which can hardly reflect the full landscape of total tumor burden. Prior work has shown that volumetric quantification of the tumor tissue at multiparametric magnetic resonance imaging (MRI) can predict survival of HCC patients2,3. However, these studies used a semi-automatic tumor segmentation approach, limiting the widespread use of volumetric parameters in clinical practice. In the present study, we aimed to evaluate the role of three-dimensional (3D) quantitative tumor burden analysis using fully automatic segmentation at MRI in the subcategorization of the BCLC stage A HCC after hepatectomy.METHODS: Consecutive adult (≥18 years) patients with surgically confirmed HCC classified as BCLC stage A who underwent contrast enhanced MRI within 1 month before curative resection between July 2010 and January 2022 were retrospectively recruited. For one-dimensional measurements, tumor diameter and number were assessed independently by two radiologic readers, blinded to clinical and survival information. Based on the radiological tumor size and number, tumor burden score (TBS) was calculated according to the following formula: TBS2 = (maximum tumor diameter) 2 + (number of tumors) 2. For 3D quantitative analysis, liver volume (cm3), total tumor volume (TTV, cm3), enhancing tumor volume (ETV, cm3), total tumor burden (TTB, %), and enhancing tumor burden (ETB, %) were obtained at preoperative MRI by using a fully automatic 3D tumor segmentation software. The model was trained using a sequential modular approach. Firstly, A 3D U-net-based algorithm was used to segment the liver in the portal venous phase automatically4. Thereafter, a unified multi-sequence lesion detector model based on Mask RCNN was developed to obtain the lesion's bounding box5. Additionally, a dynamic unfixed receptive field (RF) model that adapts the RF by adaptively integrating features with different RFs was constructed to improve adaptive perception capability at the neuron level6. Finally, a 3D U-Net based deep learning model was trained to segment liver lesions on MRI enhanced images using bounding boxes, and the output data was the predicted lesion segmentation results. The 3D diameter line of the lesion was generated using the minimum volume bounding box algorithm, and the volume of all positive voxels predicted by the model was used to calculate the volume of the liver and the lesion. TTB and ETB was defined as the ratio of TTV and ETV to the liver volume, respectively. The prognostic value of all preoperative clinical, laboratory and radiological parameters was assessed by the univariable and multivariable Cox regression analyses. Overall survival (OS) was estimated by the Kaplan-Meier method and the log-rank test.
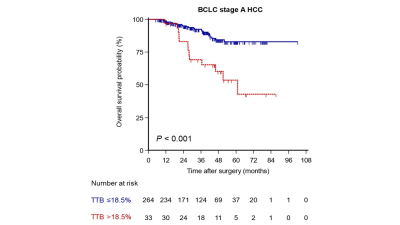
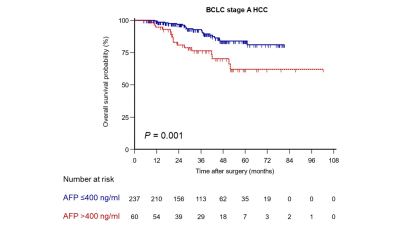
RESULTS: A total of 297 patients (age, 54.8 ± 10.8 years; 260 men) were included. After a median follow-up of 38.6 months, 5-year OS after curative resection of BCLC stage A HCC were 78.5%. The univariable Cox regression analysis identified eight preoperative variables, including maximum tumor diameter, TBS, TTV, ETV, TTB >18.5%, ETB >12.4%, age and serum AFP >400 ng/ml, as significant predictors for OS. In the multivariable Cox regression analysis, only TTB >18.5% (Hazard ratio [HR] = 2.82; P = 0.005) and serum a-fetoprotein (AFP) >400 ng/ml (HR = 2.05; P = 0.041) were independently associated with worse OS. OS of patients with TTB >18.5% was significantly shorter than that of those with TTB ≤18.5% (5-year OS rate, 53.6% vs. 82.9%; P <0.001) (Figure 1). Likewise, OS of patients with serum AFP >400 ng/ml was significantly shorter than that of those with AFP ≤400 ng/ml (5-year OS rate, 62.1% vs. 84.0%; P = 0.001) (Figure 2). Furthermore, the frequencies of microvascular invasion (MVI) and poor tumor differentiation in patients with TTB >18.5% were significantly higher than that in those with TTB ≤18.5% (MVI: 87.9% vs. 37.1%, P <0.001; poor tumor differentiation: 57.6% vs. 26.5%, P <0.001).
DISCUSSION: Our study demonstrated that 3D quantitative total tumor burden and serum AFP were independent predictors of OS and could be used to subcategorize the BCLC stage A HCC. Compared with one-dimensional measurements, volumetric quantification of TTB in relation to the liver volume was a stronger prognostic instrument for HCC patients. Moreover, using fully automatic tumor segmentation technique enables a quick estimation of the TTB while additionally reducing the known interreader variability of manual measurements. To the best of our knowledge, this is the first study in the literature to utilize fully automatic 3D segmentation-derived TTB to identify different prognostic subgroups within BCLC stage A HCC. Once validated in a larger population, it can be utilized as a pragmatic clinical tool that would help to refine the prognostic classification of the current BCLC stage A HCC.
CONCLUSION: 3D quantitative TTB using fully automatic segmentation approach at MRI and serum AFP can serve as new prognostic biomarkers for subcategorization of the BCLC stage A HCC after curative resection.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81971571, 82101997, 81901700).References
1. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76(3):681-693.
2. Borde T, Nezami N, Laage Gaupp F, et al. Optimization of the BCLC Staging System for Locoregional Therapy for Hepatocellular Carcinoma by Using Quantitative Tumor Burden Imaging Biomarkers at MRI. Radiology. 2022;304(1):228-237.
3. Jeon SK, Lee DH, Park J, et al. Tumor volume measured using MR volumetry as a predictor of prognosis after surgical resection of single hepatocellular carcinoma. Eur J Radiol. 2021;144:109962.
4. Han X, Wu X, Wang S, et al. Automated segmentation of liver segment on portal venous phase MR images using a 3D convolutional neural network. Insights Imaging. 2022;13(1): 26.
5. He K, Gkioxari G, Dollar P, et al. Mask R-CNN. IEEE Trans Pattern Anal Mach Intell, 2020;42(2): 386-397.
6. Zhou Z, Fan N, Yang K, et al. Adaptive ensemble perception tracking. Neural Netw, 2021;142: 316-328.