0791
TPA:Two-stage Progressive Attention diagnosis framework for hepatocellular carcinoma segmentation on Dynamic Contrast Enhanced MRI
Lei Lei Gao1 and Yuan-Cheng Wang1
1Zhongda Hospital Southeast University, Nanjing, China
1Zhongda Hospital Southeast University, Nanjing, China
Synopsis
Keywords: Liver, Cancer, Deep learning
We proposed a Two-stage Progressive Attention (TPA) framework by simulating the radiologists’ decision process for hepatocellular carcinoma segmentation. The study included 400 HCC patients as an internal set and 109 patients as an external test set. To obtain sensitive and specific results, our model is divided into two stages, respectively introducing attention mechanism and residual network.TARGET AUDIENCE
This study is to develop a deep learning model for automatic segmentation of hepatocellular carcinoma (HCC) lesion based on MRI, and is expected to provide an accurate prerequisite for subsequent quantitative analysis and clinical diagnosis.PURPOSE
At present, the generalization of HCC segmentation model based on MRI is not generalized because of the rich dynamic information of HCC contained in DCE-MRI cannot be completely identified. In this study, we propose a Two-stage Progressive Attention (TPA) framework by simulating the radiologists’ decision process to improve the generalizability of the model.METHODS
The retrospective study included 400 pathologically confirmed primary HCC patients who underwent liver MRI scanning. Then, the data was randomly split into a training set (240 cases), a validation set (80 cases) and an internal test set (80 cases). In addition, we further included 109 HCC data as an external test set for this study. As to the model framework, the TPA first adopted the nnU-Net framework for liver prediction1. Then, to simulate the preliminary observation of radiologists, we applied a shallow 3D U-net based Basic module to extract spatial domain feature from each DCE phase. Furthermore, To ameliorate the sensitivity of the first stage of framework, an attention mechanism was designed to combine the four results from those shallow 3D U-nets above with proper weights for each. The segmented region generated above was considered as an initial ROI for second stage, we stack multi-phase images as multiple channels of the network input. Meanwhile, we also introduced subtracted images and another attention mechanism in order to gain higher specificity and accuracy.RESULTS
For HCC segmentation, the proposed deep model, which achieved a Dice coefficient of 0.81, outperformed the 3D U-net model(Dice:0.68), and had comparable performance with the nnU-net model and LSTM model(Dice:0.83) in the internal test set. Moreover, a special dataset was collected which included: (1)The cases from external test set; (2)The population cases without late arterial phase; (3)The cases with inconsistent enhancement in late arterial phase and portal vein phase; (4)The cases whose sequences cannot be registered. In this dataset, our network reached a Dice of 0.78 and was significantly better than nnU-net(Dice:0.2), which might be contributed by the soundness of the current frame design and the ability of the model to extract rich inter-slice and time domain information.DISCUSSION
Deep learning model can effectively reduce labeling time, save labeling consumption, and improve labeling accuracy for liver cancer segmentation. At present, the segmentation model based on a single center has achieved good results2. However, the problem that the model cannot be generalized has not been effectively solved3. Our model compared different situations on MRI, simulated clinical decision-making steps, and effectively solved the problem that the early artery stage and late artery stage images cannot be registered and sometimes the enhancement is not obvious. In addition, we compared the segmentation results and found that there are several types of cases whose features are hard to be learnt by neural network and turn out with unsatisfied results: (1)The cases with small lesions less than 5mm, which image performance is not obvious; (2) The cases with hepatic perfusion in arterial phase, which is similar to HCC; (3) The cases that have adopted other surgical treatment; Although these kinds of problems only account for a small proportion, we still looking forward to solving them in the future.CONCLUSION
We introduced a deep learning model for automatic HCC segmentation. This method used 3D CNN, attention mechanism and residual network to achieve satisfactory segmentation results.Acknowledgements
NoneReferences
1.Isensee, F., Jaeger, P.F., Kohl, S.A.A. et al. nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation. Nat Methods 18, 203–211 (2021). https://doi.org/10.1038/s41592-020-01008-z 2.G. Chlebus, H. Meine, N. Abolmaali, and A. Schenk, “Automatic Liver and Tumor Segmentation in Late-Phase MRI Using Fully Convolutional Neural Networks,” in Proc. CURAC, 2018. 3.R. Zheng et al., "Automatic Liver Tumor Segmentation on Dynamic Contrast Enhanced MRI Using 4D Information: Deep Learning Model Based on 3D Convolution and Convolutional LSTM," in IEEE Transactions on Medical Imaging, vol. 41, no. 10, pp. 2965-2976, Oct. 2022, doi: 10.1109/TMI.2022.3175461.Figures
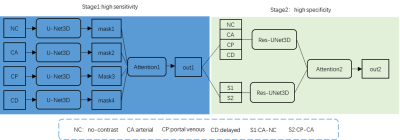
Figure 1. Overall framework of the proposed
deep learning model for HCC segmentation, two stage including a 3D U-Net module
residual block and attention mechanism.
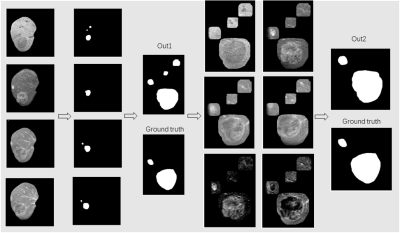
Figure 2. The visual examples of the HCC
segmentation, where the white denotes the HCC and the black denotes the
background. From left to right: Cropped liver area with HCC, multi-modality segmentation
results, sensitive out1, Smaller areas inputs by concatenating out1,final
result.
DOI: https://doi.org/10.58530/2023/0791