0786
Can HR-VWI pre-operatively predict Postoperative Restenosis of Intracranial Atherosclerotic Disease Treated by Drug-Coated Balloon?1The First Affiliated Hospital of Shandong First Medical University, Jinan, China, 2GE Healthcare, Nanjing, China
Synopsis
Keywords: Stroke, Stroke
This study mainly investigated if high-resolution vessel wall MRI (HR-VWI) can predict postoperative restenosis before drug-coated balloon (DCB) treatment. We included 76 patients who underwent HR-VWI examination before DCB treatment. DSA measurement was assessed 6 months after operation to determine vessel restenosis, classifying patients into three groups of no stenosis, mild stenosis (<50%), and restenosis (>50%). Significant differences among three groups were observed in plaque length, lumen area of MLN, degree of stenosis, enhancement amplitude and plaque burden. Plaque length and EA were independent prognostic factors of postoperative restenosis. Therefore, HR-VWI has potential to predict postoperative restenosis before DCB treatment.Introduction
Despite of aggressive clinical management, intracranial atherosclerotic disease (ICAD) remains a high risk of recurrent symptoms, high morbidity and high mortality[1]. At present, drug-coated balloon (DCB) treatment has effectively reduced restenosis via direct delivery of antiproliferative agents into the vessel wall without a permanent implant required in patients with ICAD after endovascular treatment[2-5].High-resolution vessel wall MRI (HR-VWI), allowing a direct visualization of vessel wall, permits an assessment of both luminal stenosis and vessel wall characteristics and further shows possibility of analyzing risk factors associated with postoperative restenosis.
Therefore, the main purpose of this study was to assess whether vessel wall and plaque features on pre-operative HR-VWI contributes to evaluating postoperative restenosis for ICAD patients with DCB treatment.
Materials and Methods
Subjects70 patients (aged between 22 and 75 years old) with 72 lesions were included in the study. All patients with intracranial atherosclerotic stenosis ≥ 70% or occlusion were confirmed by DSA and received HR-VWI scans 1 to 3 days prior to DCB treatment. Postoperative follow-up DSA measurement was performed after 6 months.
MRI experiments
A 3.0-T MRI scanner (GE Discovery 750w, USA) with a 32-channel head coil was used. All patients underwent HR-VWI with and without contrast agent administration. Contrast agent (Magnevist, Bayer, Germany) was used as the contrast medium (0.2 mL/kg, intravenous injection).
Image Analysis
All MRI data were transferred to the workstation (ADW4.6, GE, USA) for post-processing analysis. Multi-planar images were reconstructed along the route of vessel. Regions of interest (ROI)s were manually delineated and the corresponding characteristics were measured, including plaque signal intensity before and after enhancement, plaque length, vessel area, lumen area of maximal lumen narrowing (MLN) and the reference layer, defined as lesion-free or minimally diseased segments proximal or distal to the stenosis. The following formulas were used to calculate the parameters: (1) vessel wall area = vessel area - lumen area ; (2) plaque area = vessel wall area of MLN - vessel wall area of reference layer; (3) degree of stenosis = 1- lumen area of MLN / lumen area of reference layer; (4) plaque burden = vessel wall area / vessel area; (5) remodeling index = vessel area of MLN / vessel area of the reference layer; (6) enhancement amplitude = (plaque signal intensity after enhancement - plaque signal intensity before enhancement) / plaque signal intensity before enhancement.
Statistical Analysis
All statistical analyses were performed with GraphPad Prism (Version 9, GraphPad Software, USA). The Kruskal Wallis H-test was utilized to evaluate lumen area of MLN, plaque length and area, remodeling index, plaque burden and enhancement amplitude among three groups. Comparisons of these parameters between each two of three groups were performed using the Mann-Whitney U-test. Receiver operating characteristic (ROC) curve analysis and the area under the curve (AUC) were used to evaluate the diagnostic efficacy of HR-VWI characteristics and clinical factors in predicting postoperative restenosis. Multivariable linear regression analysis was performed to determine if any variable was independently associated with clinical outcomes. P value < 0.05 was considered statistically significant.
Results
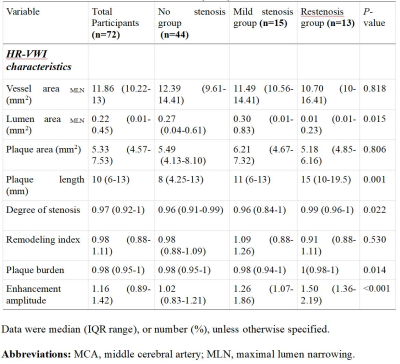
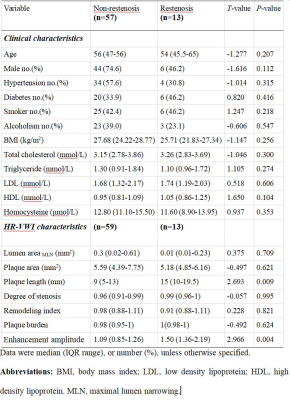
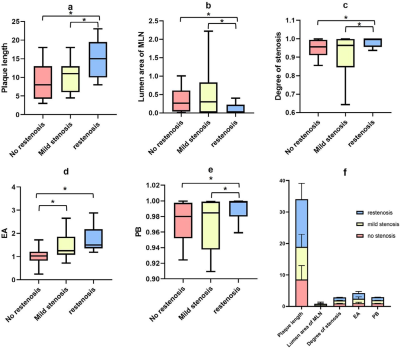
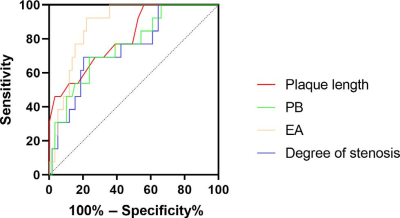
Statistically significant differences were found in lumen area of MLN, plaque length, degree of stenosis, plaque burden, and enhancement amplitude among the no stenosis, mild stenosis and restenosis groups (all p<0.05) (Table 1). With Mann-Whitney U-tests, significant differences were separately found in enhancement amplitude between no stenosis and mild stenosis groups, and between no stenosis and restenosis groups (both p<0.05). Moreover, plaque length, lumen area of MLN, degree of stenosis and plaque burden between no stenosis and restenosis groups, and between mild stenosis and restenosis groups also showed statistical significance (all p<0.05) (Table 2 and Figure 1). In ROC analysis, high AUC values were obtained for plaque length (AUC = 0.809), degree of stenosis (AUC = 0.746), enhancement amplitude (AUC = 0.880) and plaque burden (AUC = 0.759) (Figure 2). In multivariable linear regression analysis, plaque length and enhancement amplitude were independently associated with postoperative restenosis (β = 0.325 and 0.348).Discussion and conclusions
In this study, characteristics of intracranial plaque derived from pre-operative HR-VWI were used to investigate the potential in predicting DCB postoperative restenosis. Lumen area of MLN and degree of stenosis represented the atherosclerotic narrowing of vessels. High degrees of inflammation in vessels with high percentages of stenosis lead to an increased restenosis rate. Plaque burden is the ratio of vessel wall area to vessel area, reflecting the tension of vessel wall. The reduction in DCB drug absorption due to high vessel tension and insufficient hemodynamic perfusion. Plaque length, as an important parameter depicting plaque morphology, reflects unstable plaque inflammation[6]. In longer lesions, flow can be impaired by not only plaque length, but also increasing blood turbulence in the lumen by frequent presence of irregularities, curves, angulations, and bifurcations[7]. Enhancement amplitude serves as a marker for neovascularization, inflammation, and plaque instability. The enhanced permeability of endothelial cells lead to leakage of contrast agent easily[8-11]. The multivariable regression analysis revealed that plaque length and enhancement amplitude were independent predictors of prognosis and showed robust diagnostic efficiency in postoperative restenosis after DCB treatment.In conclusion, HR-VWI with vessel wall and plaque characteristics has potential to predict postoperative restenosis before DCB treatment.
Acknowledgements
NoReferences
[1] Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. 2014;45(3):663–9.
[2]Wei Zhao, Xi Chu, Yun Song, Jinping Zhang, et al. Drug-Coated Balloon Treatment for Delayed Recanalization of Symptomatic Intracranial Artery Occlusion. Transl Stroke Res. 2022 Apr 23.
[3] Philipp Gruber, Christina Braun, Timo Kahles, et al. Percutaneous transluminal angioplasty using the novel drug-coated balloon catheter SeQuent Please NEO for the treatment of symptomatic intracranial severe stenosis: feasibility and safety study. J Neurointerv Surg. 2019 Jul;11(7):719-722.
[4] Alvin Yi-Chou Wang, Chien-Hung Chang, Ching-Chang Chen, et al. Leave Nothing Behind: Treatment of Intracranial Atherosclerotic Disease with Drug-Coated Balloon Angioplasty. Clin Neuroradiol. 2021 Mar;31(1):35-44.
[5] Ryu CW, Jahng GH, Kim EJ, Choi WS, Yang DM, et al. High resolution wall and lumen MRI of the middle cerebral arteries at 3 tesla. Cerebrovasc Dis. 2009;27(5):433-42.
[6] Wendong Tang, Xiaxian Shen, Hailing Li, Yuan Bai, Bili Zhang, Zhifu Guo, Hong Wu, Pan Li, Xianxian Zhao. The independent and incremental value of ultrasound carotid plaque length to predict the presence and severity of coronary artery disease: analysis from the carotid plaque length prospective registry. Eur Heart J Cardiovasc Imaging. 2020 Apr 1;21(4):389-396.
[7]Kastrati A, Dibra A, Mehilli J, et al. Predictive factors of restenosis after coronary implantation of sirolimus- or paclitaxel-eluting stents. Circulation 2006;113:2293–2300.
[8] Yue Lu, Meng-Fan Ye, Jie-Ji Zhao, Shan-Shan Diao, Tan Li, Dong-Xue Ding, Lu-Lu Zhang, Fei-Rong Yao, Yan Kong, Zhuan Xu. Gadolinium enhancement of atherosclerotic plaque in the intracranial artery. Neurol Res 2021; 43: 1040-1049.
[9] Liu S, Tang R, Xie W, Chai S, Zhang Q, Luo Y, Guo Y, Chai C, Huang L, Zheng M, Zhu J, Chang B, Yang Q, Jin S, Fan Z and Xia S. Plaque characteristics and hemodynamics contribute to neurological impairment in patients with ischemic stroke and transient ischemic attack. Eur Radiol 2021; 31: 2062-2072.
[10] Shu-Jia Zhai, Lin Jia, Han-Jiaerbieke Kukun, Yun-Ling Wang, Hong Wang, Shuang Ding, Wen-Xiao Jia. Predictive power of high-resolution vessel wall magnetic resonance imaging in ischemic stroke. Am J Transl Res. 2022 Jan 15;14(1):664-671.
[11] Bing Tian, Chengcheng Zhu, Xia Tian, Qinqin Kang, Chengwei Shao, Mahmud Mossa-Basha, Jianping Lu, David A Saloner. Baseline vessel wall magnetic resonance imaging characteristics associated with in-stent restenosis for intracranial atherosclerotic stenosis. J Neurointerv Surg. 2022 Mar 1;neurintsurg-2021-018473.
Figures