0776
Accelerated intracranial time-of-flight magnetic resonance angiography using wave-encoding1Wellcome Centre for Integrative Neuroimaging, FMRIB Division, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom
Synopsis
Keywords: Blood vessels, Parallel Imaging
3D time-of-flight (TOF) can be used to acquire a volume with high spatial resolution, making it a preferable choice for depicting smaller vascular structures. However, 3D TOF requires long acquisition times when acquiring multiple slabs and covering a large field of view, especially for high spatial resolution imaging. To accelerate acquisition and to improve image quality of TOF MRA, we developed an accelerated 3D intracranial TOF MRA sequence with wave-encoding (referred to as 3D wave-TOF) and evaluated two variants – wave-CAIPI and compressed-sensing wave (CS-wave).Introduction
TOF is a widely used non-contrast-enhanced magnetic resonance angiography technique, which utilizes the magnetization difference between unsaturated spins of inflowing blood and saturated stationary spins to enhance blood vessels 1. However, the main disadvantages of 3D-TOF MRA are the long acquisition times when acquiring multiple slabs and covering a large field of view, especially for high spatial resolution imaging. Wave-controlled aliasing in parallel imaging (wave-CAIPI) is an emerging parallel imaging technique 2, which has been demonstrated to achieve highly accelerated 3D volume imaging with low artifact and SNR penalties. In this work, we evaluated the feasibility of combining 3D-TOF MRA with the wave-CAIPI technique to accelerate imaging speed and to improve the imaging quality of the cerebral vasculature. CS was also combined with wave-encoding (dubbed as CS-wave), and the performance of both wave-CAIPI and CS-wave TOF was evaluated.Methods
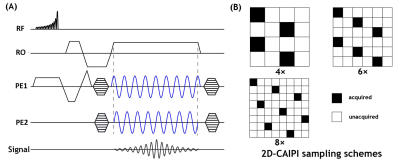
Sequence implementation and data acquisitionA schematic diagram of the proposed 3D wave-TOF sequence is shown in Figure 1A. The sequence was implemented based on a standard 3D-TOF sequence with additional sinusoidal gradients applied in both phase and partition encoding directions. Figure 1B shows three 2D-CAIPI sampling patterns 3.
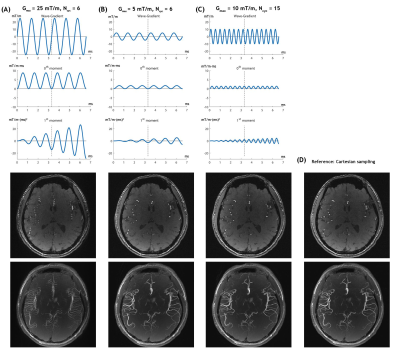
All MRI measurements were performed on a 3T Siemens MAGNETOM Prisma scanner. Retrospectively and prospectively undersampled datasets were acquired using the proposed 3D wave-TOF and conventional Cartesian 3D-TOF MRA. The common parameters used in sequences studied were as follows: matrix size per slab = 256×256×44, resolution = 0.8×0.8×0.6 mm3, TR/TE=14/3.5 ms, flip angle = 20°, and bandwidth = 121 Hz/pixel. To avoid flow-related artifacts caused by the wave-encoding gradients, several sets of the wave-encoding parameters were evaluated and a cycle number (Ncyc) of 15 and wave amplitude (Gmax) of 10 mT/m were finally chosen for the wave-TOF MRA protocol.
Reconstruction
Images were reconstructed offline from the raw k-space data with an in-house program coded in MATLAB. Both retrospectively and prospectively undersampled wave-CAIPI data were reconstructed by solving the following minimization problem:$$min_s ‖M⋅F_z ⋅F_y⋅PSF(k_x,y,z)⋅F_x⋅C⋅s-k‖_2^2$$ where M is the mask of the 2D-CAIPI sampling pattern; Fx, Fy and Fz are the Fourier transform operators; $$$PSF(k_x,y,z)$$$is the point spread function of the wave-encoding gradients; C is the estimated coil sensitivity; s is the unknown image to be reconstructed; and k is the undersampled wave-encoded k-space data. Similarly, the undersampled Cartesian data were reconstructed using the SENSE algorithm, but without the PSF term. The images were then recovered from the undersampled CS-wave dataset by solving the following optimization problem 4,5:$$min_s ‖M⋅F_z ⋅F_y⋅PSF(k_x,y,z)⋅F_x⋅C⋅s-k‖_2^2+γ_G ‖G_s ‖_1+γ_W ‖W_s ‖_1$$where Gs and Ws denote the gradient and Haar wavelet transforms of s, respectively. γG and γW are the regularization weights. Reconstruction of the sub-sampled Cartesian dataset was performed with the CS algorithm, but without the PSF term.
Image analysis
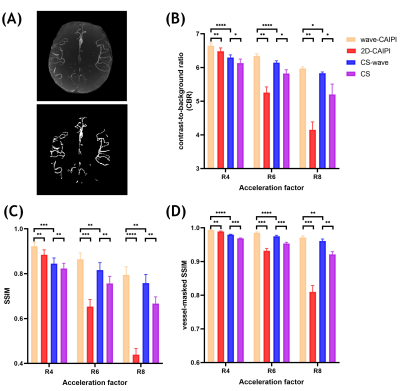
Contrast-to-background ratio (CBR) between cerebral arteries and tissue background 6 in source images was measured to evaluate the visibility of vessels over the background with various acceleration approaches. we also adopted the vessel-masked structural similarity (SSIM) index in addition to SSIM to assess the accuracy of the reconstruction from retrospectively undersampling.
Results
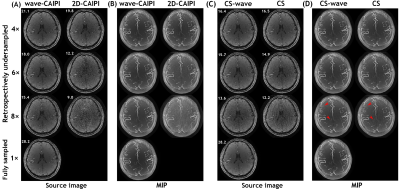
Figure 2 shows the evolutions of the 0th and 1st order moment of the wave-encoding gradient for different values of Ncyc and Gmax during the readout period, along with a source image of a representative slice and a maximum intensity projection (MIP) image of a slab obtained by the wave-TOF sequence.To visualize the performance of the wave-CAIPI technique in TOF MRA, comparisons between the images from accelerated wave-CAIPI TOF and conventional TOF are shown in Figures 3A and 3B. As depicted in the figures, the wave-CAIPI technique demonstrates its superiority for vessel contrast, and the robustness of wave-CAIPI to noise is an obvious advantage compared to a conventional imaging scheme. Next, we exploit the integration of wave-encoding, random sampling, and CS reconstruction with a sparsity prior (CS-wave) to further accelerate the acquisition and improve the image quality of intracranial TOF MRA. Figures 3C and 3D compare the results of retrospectively accelerated imaging schemes using CS-wave and traditional CS for intracranial TOF MRA.
Figure 4A shows an example of a MIP image and the corresponding vessel mask which was used for calculating the CBR and the vessel-masked SSIM. Figure 4B shows the quantitative analyses of the CBR between vessels and static tissue background. Figure 4C and 4D show the SSIM and vessel-masked SSIM between the MIP images obtained from the undersampled reconstruction and the reference dataset from the fully sampled reconstruction without and with vessel masking, respectively.
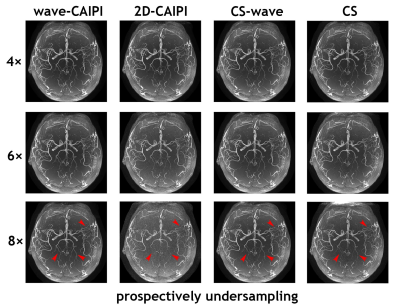
Figure 5 shows an example of the whole-brain axial MIP images obtained from all four slabs with various prospectively undersampled schemes: wave-CAIPI, 2D-CAIPI, CS-wave, and conventional CS at various acceleration factors. Visually, 2D-CAIPI performs the worst at high acceleration factors with an obvious noisy background when compared with other sampling schemes with the same acceleration factor.
Discussion and Conclusion
The flow-related artifacts caused by the wave-encoding gradient can be significantly suppressed by decreasing the oscillation amplitude of the 1st order gradient moment. 3D wave-TOF improves the capability of accelerated MRA and provides better image quality at higher acceleration factors compared to traditional PI- or CS-accelerated TOF, suggesting the potential use of wave-TOF in cerebrovascular disease.Acknowledgements
This study was supported by the Oxford NIHR Biomedical Research Centre. The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z). We also thank the Dunhill Medical Trust (PJ, MdB), Royal Academy of Engineering (RF201819/18/92, WW) and grant support from Siemens Healthineers (MdB). TO is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (220204/Z/20/Z).References
1. Parker DL, Yuan C, Blatter DD. MR angiography by multiple thin slab 3D acquisition. Magnetic Resonance in Medicine 1991;17(2):434-451.
2. Bilgic B, Gagoski BA, Cauley SF, Fan AP, Polimeni JR, Grant PE, Wald LL, Setsompop K. Wave‐CAIPI for highly accelerated 3D imaging. Magnetic Resonance in Medicine 2015;73(6):2152-2162.
3. Breuer FA, Blaimer M, Mueller MF, Seiberlich N, Heidemann RM, Griswold MA, Jakob PM. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magnetic Resonance in Medicine 2006;55(3):549-556.
4. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magnetic Resonance in Medicine 2007;58(6):1182-1195. 31.
5. Bilgic B, Ye H, Wald LL, Setsompop K. Simultaneous time interleaved multislice (STIMS) for rapid susceptibility weighted acquisition. Neuroimage 2017;155:577-586. 6. Yamamoto T, Fujimoto K, Okada T, Fushimi Y, Stalder AF, Natsuaki Y, Schmidt M, Togashi K. Time-of-flight magnetic resonance angiography with sparse undersampling and iterative reconstruction: comparison with conventional parallel imaging for accelerated imaging. Investigative Radiology 2016;51(6):372-378.
Figures