0775
BB MRI for monitoring aneurysm treatment – a novel biomarker?1Department of Radiology and Neuroradiology, Section Biomedical Imaging, Molecular Imaging North Competence Center (MOIN CC), University Medical Center Schleswig-Holstein (UKSH), Kiel University, Kiel, Germany, Kiel, Germany, 2Department of Radiology and Neuroradiology, University Medical Center Schleswig-Holstein (UKSH), Kiel University, Kiel, Germany, Kiel, Germany, 3Research Campus STIMULATE, University of Magdeburg, Magdeburg, Germany, Magdeburg, Germany
Synopsis
Keywords: Blood vessels, Phantoms, blood vessels, aneurysms, diagnostics, treatment evaluation
Treating life-threatening intracranial aneurysms with flow modulating devices (FMDs) is mostly successfull, but may lead to complications and even delayed aneurysm rupture. A biomarker indicating treatment success is direly needed. Flow MRI can detect flow reduction, but it is impaired by metal artifacts from FMDs. Black-blood (BB) MRI is less sensitive to metal artifacts and often results in poor slow-flow suppression. We hypothesize that BB contrast changes after aneurysm treatment. Here, after placing FMDs in 3D-printed aneurysm models, an enhanced signal was found on BB MRI, associated with the reduced aneurysmal flow after flow modulation and thus possible treatment success.Introduction:
Intracranial aneurysms (IAs) are life-threatening diseases that can be treated with flow-modulating devices (FMDs) by reducing the flow into the aneurysm. However, IA may still rupture after the treatment, and thus a biomarker indicating treatment success is needed.Flow MRI was used to quantify the flow reduction after treatment1 and to predict its succes2. However, flow MRI is impaired by metal artifacts originating from FMDs.
Black-blood (BB) MRI is less sensitive to metal artifacts and does not suppress slow flow well. High BB signal in the aneurysm lumen before treatment was associated with insufficient flow suppression and with slow-flowing blood3.
Here, we aim to investigate whether the BB signal in IAs changes after FMD treatment and could be used as a biomarker for treatment success. To this end, we assessed flow velocities and BB signal before and after placing FMDs in vitro using 3D-printed aneurysm models.
Methods:
Aneurysm models were constructed in house4 based on patient 3D rotational angiographic data. In total, six bifurcation tip aneurysm models were constructed (model 1 (N=2) and model 2 (N = 4) with aneurysm widths 6.7 and 2.8 mm, respectively, Fig. 1a). The models were 3D printed with stereolithography, submerged in an agarose gel, and connected to a pulsatile pump with a mean flow of 150 ml/min. Aneurysm treatment was performed by placing intrasaccular devices with a diameter of 5 and 11 mm into models 1 and 2, respectively (contour neurovascular system, contour-5 (N=2) and contour-11 (N=4), Fig. 1b-c).MRI measurements were performed on 3T MR system with a 32-channel head coil (Ingenia CX, Philips Healthcare). A T1-weighted (T1w) BB 3D variable refocusing flip angle turbo spin echo sequence (TE/TR: 28/700 ms; FOV: 200×250×160 mm3, voxel size: (0.65 mm)3, echo train length: 55). Time-resolved phase-contrast (4D flow) MRI was acquired with 3D velocity encoding T1w spoiled fast gradient echo sequence with Cartesian sampling (TE/TR: 5/8.5 ms; FOV: 100×110×40 mm3; voxel size: (0.75 mm)3; max. velocity-encoding: 75 cm/s, 20 cardiac phases).
MRI data analysis: 4D flow MRI was analyzed as follows: a) five equidistant slices perpendicular to the aneurysm and the parental artery were created; b) a region-of-interest (ROI) was manually drawn around the vascular lumen on each slice; c) the flow within a ROI was calculated, averaged over slices and time. TOF MRI was used to select the volume (3D ROI) where the BB signal was evaluated (Fig. 2). After that, the BB signal was normalized by the signal of agarose gel.
Blood flow simulations were done using computational fluid dynamics. Virtual vessels were based on 3D-printed aneurysm models. Inlet and outlet boundary conditions were set according to measured flow and pressure, respectively.
Results:
4D flow MRI of the models was successfully acquired with and without FMDs. The FMDs caused strong metal artifacts at the neck of the aneurysm (Fig. 3a-b, black and yellow arrows) and reduced the flow in the aneurysm (0.08 vs. 0.05 ml/min without and with FMD, respectively, for model 1; 3.4 vs. 0.3 ml/min without and with FMD, respectively, for model 2). Flow at the parental vessel was similar regardless of the presence of the device (Fig. 4). Flow at model 1 was low with and without FMD. This was confirmed with flow simulations reproducing the experiments with higher spatial and temporal resolution.Likewise, the BB signal in the aneurysm lumen was increased after placing the FMDs (0.8 vs. 1.4 a.u. without and with FMD, respectively, for model 1; 0.1 vs. 4.5 a.u. without and with FMD, respectively, for model 2), while it was similar at the parental vessel (Fig. 5). The effect was reproducible among all models and devices. The increased BB signal was colocalized with the reduced flow (Fig. 3). Moreover, the metal artifacts were less apparent in the BB images compared to 4D flow.
Discussion:
In our previous work, we observed that slow flow in aneurysms led to bright BB MRI signal3. Now, we observed a similar effect after treatment. This effect is likely a consequence of reduced aneurysmal flow and poor slow-flow suppression of BB MRI. While “failing” BB MRI is usually considered an artifact, it may be a valuable tool to assess flow reduction after aneurysm treatment, which is more robust to metal artifacts and much faster than 4D flow MRI2. Indeed, BB MRI allowed imaging of the lumen of a coiled aneurysm (with a coil in the lumen)5. However, BB MRI is not quantitative, and care must be taken as different BB parameters will lead to different flow suppression. In vivo studies are needed to evaluate the clinical relevance and feasibility of BB MRI to assess flow reduction and aneurysm treatment outcomes.Conclusion:
Increased intra-aneurysmal BB MRI signal was observed in aneurysm flow models after flow modulation and was associated with reduced flow. Spin echo-based BB MRI was less sensitive to metal artifacts compared to 4D flow MRI. Therefore, BB-MRI might provide a clinically applicable non-invasive marker of aneurysm flow reduction and successful aneurysm treatment.Acknowledgements
We acknowledge support by the RTG "Materials4Brain" (GRK2154; P2), Forschungscampus STIMULATE (13GW0473A), and the German Research Foundation (SPP2311, project number: 441884911).References
1. Pereira, V. M. et al. Assessment of intra-aneurysmal flow modification after flow diverter stent placement with four-dimensional flow MRI: a feasibility study. J. NeuroInterventional Surg. (2015), doi:10.1136/neurintsurg-2014-011348
2. Brina, O. et al. How flow reduction influences the intracranial aneurysm occlusion: a prospective 4D phase-contrast MRI Study. Am. J. Neuroradiol. (2019) doi:10.3174/ajnr.A6312.
3. Pravdivtseva, M. S. et al. Pseudo-enhancement in intracranial aneurysms on black-blood MRI: effects of flow rate, spatial resolution, and additional flow suppression. J. Magn. Reson. Imaging (2021), doi:10.1002/jmri.27587
4. Pravdivtseva, M. S. et al. 3D-printed, patient-specific intracranial aneurysm models: from clinical data to flow experiments with endovascular devices. Med. Phys. (2021), doi:10.1002/mp.14714
5. Larsen, N. et al. Visualization of Aneurysm Healing. Clin. Neuroradiol. 30, 811–815 (2020), doi:10.1007/s00062-019-00854-5
Figures
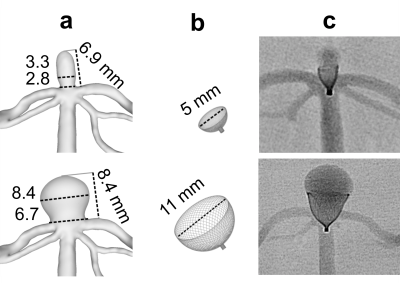
Fig. 1: 3D rendering of two different aneurysm models, model 1 (a top) and model 2 (a bottom) digital models of contour-5 and contour-11 (b), and X-ray projection of aneurysm models with implanted devices (c).
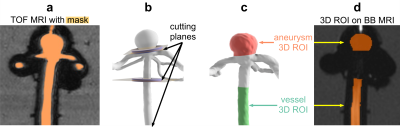
Fig. 2: BB MRI processing pipeline. A TOF MRI (a) was used to define the volumes where the BB MRI signal was quantified (d). A binary mask containing vessel lumen on TOF MRI was created (a), and the resulting vessel lumen was cut into three volumetric regions (b). The aneurysm and vessel regions (c) were translated to BB images (d).
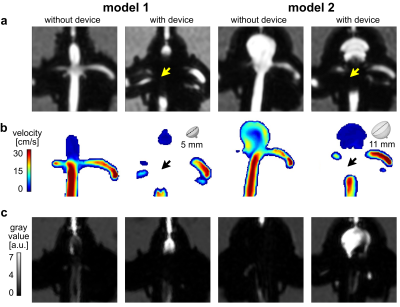
Fig. 3: Representative images of 4D flow magnitude MRI (a), velocity maps (b) and black blood MRI (c) of aneurysm models with and without FMDs. All FMDs caused strong metal artifacts at the aneurysm neck (a-b, black and yellow arrows), reduced the aneurysmal flow (b), and increased the BB signal (c).
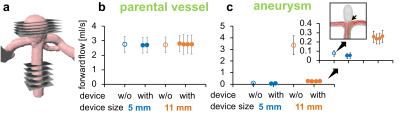
Fig. 4: Five planes were used to measure the flow in the aneurysm and parental vessel (a). The flow in the parental vessel was similar (b), while the flow at the aneurysm was strongly reduced after implanting contour-11 (c). Contour-5 did not cause a strong flow reduction, because the flow at the aneurysm was low even before implantation. This was confirmed with flow simulations reproducing the experiments with higher spatial and temporal resolution (c, inset). The effect was reproducible among different devices and models.
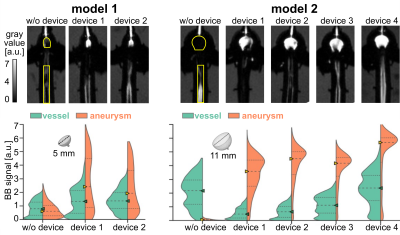
Fig. 5: BB MRI of the models with and without FMDs (top), and quantified signals in the vessel and aneurysm (bottom). The signal in the aneurysm was increased after placing the devices, while the signal in the parental vessel did not change much. The effect was reproducible among the models and devices. ROIs are indicated on top (yellow outline). Triangles and dashed lines show the median BB signal among the ROI, dotted line shows upper and lower quantiles.