0752
Experimental and numerical calibration procedure for RF safety evaluation of implant-embedded sensors1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany
Synopsis
Keywords: Safety, Data Acquisition
Embedded sensors in implants that communicate with an MRI system can improve patient safety substantially, in particular when parallel transmission is applied to mitigate the RF-heating risk. Calibration procedures and virtual models are presented in this work to support a rigorous safety assessment of this novel approach to implant safety. Experiments show a good correlation (r>0.96) between sensor signal (voltage at the tip) and field-probe measurements (E-field and temperature) for a custom-built reference implant. These results are further supported by simulations that can be extended to investigate more complex implant heating scenarios in human voxel models.Introduction
Radio-frequency (RF) caused burns by implants in MR are a major risk1. Accidents are still being reported2 even for ASTM F2503-203 MR conditional implants. Consequently, current implant safety assessment procedures are complicated and error-prone, posing a serious burden to manufacturers and clinical personnel.Combining integrated sensors in active implantable medical devices (AIMDs) with parallel transmission (pTx) capable MR scanners exhibits promising results in mitigating RF caused implant heating while maintaining imaging performance4,1,5,6.
Sensors at the implant's tip, measuring the temperature hot-spot patient-specific in-situ offer the most information7–9, but might be challenging to implement in real implant leads.
Readout electronics in the implantable pulse generator (IPG) using RMS sensors have been presented10, that can rapidly assess implant-tip heating11. This technique can in principle be applied to a variety of currently available coaxial implant lead configurations12,13. Calibration procedures to link the measured sensor signal from the implant to an established safety metric (SAR or temperature) and validated simulation models are needed for a sound safety assessment.
In this work, an experimental and simulation setup is proposed, which can be used to evaluate implant-embedded sensors and assess their uncertainties for the detection of implant-safety related parameters.
Methods
Experiments were performed with a parallel transmission (pTx) testbed at 297MHz5. A cylindrical PVP/water phantom (radius=100mm, height=198mm, $$$\varepsilon$$$=43.8, $$$\sigma$$$=0.35S/m) was positioned within a 7T 8-ch head coil14. A semi-rigid coaxial cable (SUCOFORM_141_CU_FEP, shield: 12mm uninsulated, inner conductor 5mm extended) bent into a loop served as dummy implant. Radial E-field and temperature near the tip were measured by two reference probes (E1TDSz SNI, SPEAG, not calibrated in PVP solution; FBG/FBG-TEMP-XXS with CANFDX/L-FBG-T8, imc) (Fig.1A). The implant lead was submerged into the phantom with the tip ~15mm below the liquid surface (Fig.1B). 1000 pulses with random RF amplitudes and phases were transmitted (duration: 100µs, maximum single channel voltage 0.55V to avoid E-field sensor saturation). The induced tip voltage was measured at the remote end of the lead by the ADC of the testbed5 and compared to the reference measurements from the E-field and temperature probes. For heating experiments, ten shim vectors covering the full range of RF-induced tip voltages were consecutively applied for 60s. The maximum single-channel voltage was increased to 2.75V to increase the SNR of the temperature measurements. All heating experiments were repeated for a second implant trajectory, with the tip ~50mm away from the E-field probe (Fig.1C).Simulations: An electromagnetic FDTD simulation at 297MHz with 100µm spatial resolution (volume: 44mm$$$\times$$$44mm$$$\times$$$74mm to lower computational burden) was carried out in Sim4Life 7.0 (ZMT) with the implant-tip positioned inside a rectangular phantom ($$$\varepsilon$$$=43.8,$$$\sigma$$$=0.35S/m, thermal conductivity=0.1W/m/K15) (Fig.2). Excitation was performed at the remote end of the implant-tip between outer conductor and a “ground plane” as indicated in Fig.2. The voltage across a 50$$$\Omega$$$ load between inner conductor and shield was recorded at the remote end of the implant lead and the electric loss density was used as heat source for transient thermal simulations applying Pennes’ Bioheat Equation (PBE)16 (60s heating, 60s cooling; same voxel grid as the EM simulation, shield and inner conductor at constant temperature).
Results
Sensor signal (tip-voltage measured at the remote end of the implant) and radial E-field probe (in the liquid near the implant-tip) correlate well (Pearson r=0.97, Fig.3A). Similarly, the temperature probe shows an excellent correlation to the tip voltage squared (r=0.99, Fig.3B). The temperature evolution of the RF heating experiments is shown in Fig.3C and despite a baseline temperature drift, an excellent correlation of the temperature rise (Fig.3D) and normalized max temperature (Fig.3E) is observed. All normalized temperature curves (except RF shim vector #1 with close to zero heating) show a similar time course.SAR hot spots occur primarily at the edges of inner conductor and shield (Fig.4).
The scaled simulated temperature evolution matches the measured temperature rise curve very well (Fig.5A). The highest temperatures occur at the edges of the shield and the tip of the core (Fig.5B,C). Nevertheless, the actual location of the temperature sensor is critical to determine absolute values but also for the time course of the measured temperature curve (Fig.5B).
Discussion
The calibration setup shows good correlation between measured tip E-fields and tip temperatures as well as the corresponding induced implant sensor readings that are performed within the AIMD’s IPG. A sensor in an IPG is easier to realize than a sensor embedded directly at the implants tip, which is an advantage for realistic implementations. The described approach in conjunction with wireless transfer10 could be used to enable safe, high-quality MRI scans of implant carriers5,6. The simulations correspond well to the measurements. Scaling of the simulated temperature curve was possible due to the linearity of the temperature simulation. The determined scaling factor can be used to set-up implant lead simulations with more complex lead trajectories in human voxel models to evaluate implant safety and pTx mitigation schemes of sensor-equipped implants.Conclusion
The presented experimental and numerical simulation methodology can be used to investigate implant safety on a variety of implant lead configurations in sensor-equipped implants. This is an important step towards the evaluation of a novel safety concept, where a sensor-equipped implant communicates with an MRI system to substantially improve implant safety.Acknowledgements
This work has received funding from the European Partnership on Metrology, co-financed by the European Union’s Horizon Europe Research and Innovation Programme and by the Participating States, under grant number 21NRM05 STASIS.References
- Winter L, Seifert F, Zilberti L, Murbach M, Ittermann B. MRI‐Related Heating of Implants and Devices: A Review. J Magn Reson Imaging. Published online May 26, 2020:jmri.27194. doi:10.1002/jmri.27194
- Turner S, Singh SM. Skin burn after magnetic resonance imaging in a patient with an implantable cardioverter-defibrillator. HeartRhythm Case Reports. 2022;8(7):539-540. doi:10.1016/j.hrcr.2022.05.023
- F04 Committee. Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment. ASTM International doi:10.1520/F2503-20
- Etezadi-Amoli M, Stang P, Kerr A, Pauly J, Scott G. Controlling radiofrequency-induced currents in guidewires using parallel transmit: Controlling RF Current Using Parallel Transmit. Magn Reson Med. 2015;74(6):1790-1802. doi:10.1002/mrm.25543
- Winter L, Silemek B, Petzold J, et al. Parallel transmission medical implant safety testbed: Real‐time mitigation of RF induced tip heating using time‐domain E‐field sensors. Magn Reson Med. Published online July 8, 2020:mrm.28379. doi:10.1002/mrm.28379
- Silemek B, Seifert F, Petzold J, et al. Rapid safety assessment and mitigation of radiofrequency induced implant heating using small root mean square sensors and the sensor matrix Q s . Magn Reson Med. 2022;87(1):509-527. doi:10.1002/mrm.28968
- Taber KH, Hayman LA. Temperature monitoring during MR imaging: Comparison of fluoroptic and standard thermistors. Journal of Magnetic Resonance Imaging. 1992;2(1):99-101. doi:10.1002/jmri.1880020119
- Luechinger R, Zeijlemaker VA, Pedersen EM, et al. In vivo heating of pacemaker leads during magnetic resonance imaging. Eur Heart J. 2005;26(4):376-383; discussion 325-327. doi:10.1093/eurheartj/ehi009
- Silemek B, Acikel V, Oto C, et al. A temperature sensor implant for active implantable medical devices for in vivo subacute heating tests under MRI. Magnetic Resonance in Medicine. 2018;79(5):2824-2832. doi:10.1002/mrm.26914
- Silemek B, Seifert F, Ittermann, Bernd, Winter L. Wireless reference implant and communication methodology to assess and investigate RFsafety and pTx mitigation strategies for AIMDs. In: Proc. Intl. Soc. Mag. Reson. Med. 30. ; 2022:4300.
- Silemek B, Seifert F, Brühl R, et al. Mitigation of RF-induced heating on realistic deep brain stimulator lead trajectories by wireless sensor Q-matrix and parallel transmission. In: Proc. Intl. Soc. Mag. Reson. Med. 30. ; 2022:173.
- Mond HG, Helland JR, Fischer A. The Evolution of the Cardiac Implantable Electronic Device Connector. Pacing and Clinical Electrophysiology. 2013;36(11):1434-1446. doi:10.1111/pace.12211
- Nisam S, Reddy S. The story of … a lead. EP Europace. 2015;17(5):677-688. doi:10.1093/europace/euu391
- Seifert F, Pfeiffer H, Mekle R, Waxmann P, Ittermann B. 7T 8-channel pTx head coil with high B1+ efficiency optimized for MRS. In: Proc. Intl. Soc. Mag. Reson. Med. 24. ; 2016:3545. https://index.mirasmart.com/ISMRM2016/PDFfiles/3545.html
- Dakroury AZ, Osman MBS, El-Sharkawy AWA. Thermal properties of aqueous solutions of polyvinylpyrrolidone in the temperature range 20–80°C. Int J Thermophys. 1990;11(3):515-523. doi:10.1007/BF00500843
- Pennes HH. Analysis of Tissue and Arterial Blood Temperatures in the Resting Human Forearm. Journal of Applied Physiology. 1948;1(2):93-122. doi:10.1152/jappl.1948.1.2.93
Figures
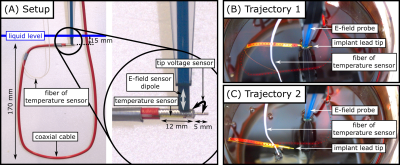
Fig.1. Experimental setup for measuring the correlation between implant-tip voltage, E-field and temperature. (A): Coaxial cable forming a loop in the phantom with attached fiber optic temperature sensor. (B): Trajectory 1 with implant-tip next to the E-field probe. (C): Trajectory 2, where the tip was bend ~50 mm from trajectory 1.
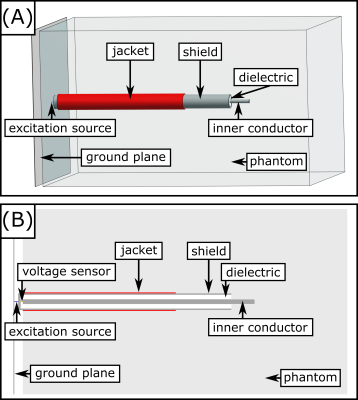
Fig.2. Simulation setup of the coaxial cable tip. (A): 3D view. (B): Slice through the central plane.
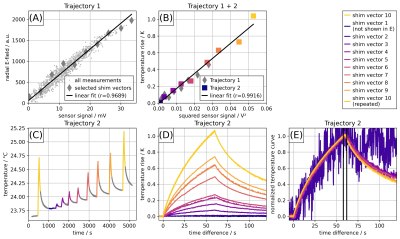
Fig.3. (A): E-field sensor signal vs. induced implant-tip voltage for 1000 random RF shim vectors. The 10 shim vectors used for consecutive RF heating experiments are labeled with a diamond. The E-field probe shows a saturation above 1500 units. (B): Temperature vs. squared implant-tip voltage for 10 RF shim vectors. The tip voltage is scaled by a factor of 5 compared to A. (C): Temperature evolution and (D): temperature rise at trajectory 2 using the 10 selected shim vectors. (E): Normalized temperature curves of the 9 shim vectors with highest signal for trajectory 2.
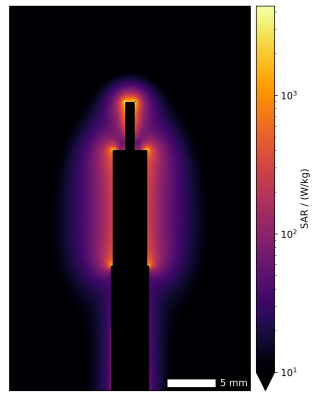
Fig.4. Point SAR in the implant-tip plane. Please note the logarithmic scale.
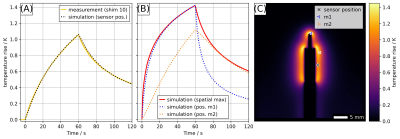
Fig.5. (A): Comparison of simulation and measurement results. Measured temperature evolution for RF shim vector #10 and simulated temperature course at the sensor position marked by "╳" scaled to the same maximum temperature rise. (B): Time course of spatial temperature maximum and temperature rise curves for position m1 and m2 marked by "⤙" and "⤚" in C. (C): Simulated temperature rise map after 60 s of heating.