0751
eGantryMate: A Piezo-Motor driven lean and flexible Assistance System for MR-Guided Interventions at 1.5T1Division of Medical Physics, Department of Radiology, University Medical Center Freiburg, Freiburg, Germany, 2Division of Interventional Radiology, Department of Radiology, University Medical Center Freiburg, Freiburg, Germany, 3Interventional Systems GmbH, Kitzbuehel, Austria
Synopsis
Keywords: Interventional Devices, Interventional Devices
We present a small and flexible piezo-driven assistance system, which can be steered from outside the magnet bore via a control unit. The assistance system is combined with a tracking sequence, which is able to follow in-bore manipulations of a dedicated end effector in real-time. For an initial evaluation, the assistance systems is equipped with a biopsy needle and targeting experiments are performed in a phantom setting.Introduction
Image-guided interventions in clinical MRI systems benefit from high SNR and good CNR at high field strengths which allows distinguishing lesions or tumors from healthy tissue. The closed-bore design, however, limits the access to the patient. In a clinical routine workflow it is often difficult to reach into the magnet bore to perform instrument alignments; hence, the patient has to be repeatedly moved in and out, and the imaging plane encompassing the instrument and the target has to be re-adjusted. This workflow is time-consuming and stressful for the physician and patient which decreases the clinical acceptance of MR-guided interventions. To overcome these limitations, remote-controlled assistance systems have been proposed that can align the instrument in the magnet1–3.In this work, we present a motorized version of a small and flexible assistance system which we combine with a passive tracking sequence. The system can be mounted directly on the patient or on a dedicated holding arm. It is driven by piezo motors steered via a control unit (CU) from outside the magnet. The combination with a tracking sequence allows to perform in-bore movements of an instrument holder for targeting procedures in real-time. We show that the assistance system does not produce image artifacts during operation inside the magnet and assess the accuracy of needle insertions in phantom experiments.
Methods
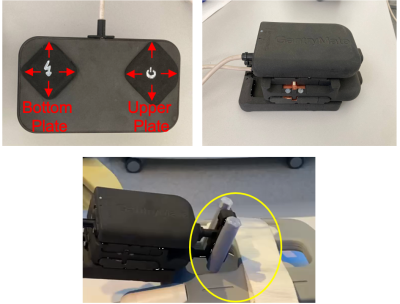
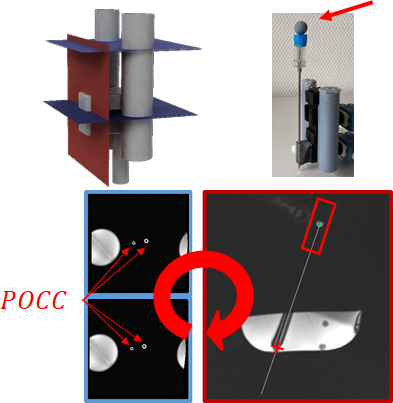
The assistance system (eGantryMate, Interventional Systems GmbH, Kitzbühel, Austria) consists of an instrument positioning unit (IPU) and a CU (Fig.1). The design of the IPU is based on a previously described mechanical version4 with three stacked plates; a base plate for gross-positioning and two sliding plates with a distal instrument holder. Each sliding plate can be individually shifted in two translational (forward-backward–FB, left-right–LR) degrees of freedom (DOF) to the respective plate below. Thus, movements of the bottom plate allow a translational motion in FB/LR direction, while the upper plate performs rotations of the instrument holder with approx. ±30°. In the piezo-driven version, each DOF is performed with linear piezo motors (Piezo LEGS, PiezoMotor, Sweden) which are powered via a battery inside the CU. The entire device is shielded and the 5m-long cable between the controller and IPU is equipped with floating resonant RF traps (BALUNs) every 80cm. Additionally, each motor channel is equipped with a low-pass suppression filter (<5MHz).For device detection, two coaxial cylinders (small/large cylinder: inner diameter:5/6.5mm, outer diameter:10/13mm) filled with a contrast agent solution (Magnevist®/H2O:1/200, Bayer Schering Pharma AG, Berlin, Germany) are used as an end-effector. Both cylinders are detected with a phase-only cross-correlation (POCC) tracking sequence5–10. Therefore, the sequence acquires two displaced cross-sectional FLASH tracking images, such that both cylinders appear as rings (Fig.2). In each tracking image, the position of the rings is automatically determined using POCC template matching. With the 4 POCC positions a plane is calculated which allows to acquire a targeting image coplanar to the needle guide (shift from cylinder plane:20mm,Fig.2). For needle tip estimation, the needle handle is equipped with a spherical marker filled with contrast agent solution which is detected in the targeting image via maximum intensity projection in a region of interest (ROI). With the known distance between the marker and the needle tip, the estimated needle tip position is projected onto the image. The sequence runs in a feedback loop and automatically follows movements of the end-effector.
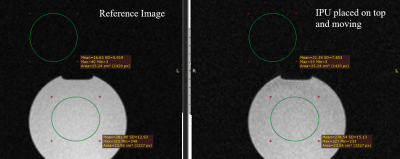
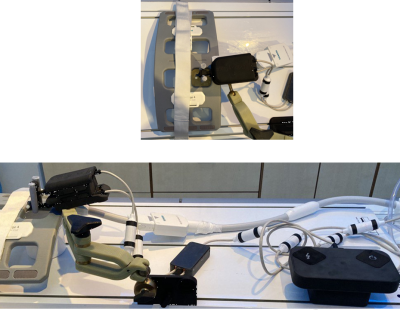
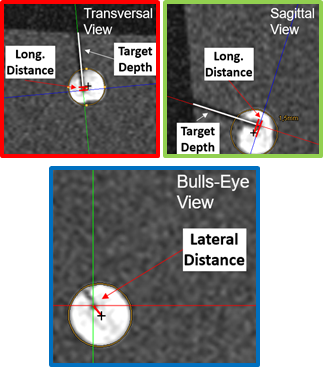
All experiments were performed in a 1.5T whole body system (Aera, Siemens Healthineers) using the vendors’ flex coil for signal reception. To detect signal interferences, GRE images were acquired without and with the device in operation (Fig.3). For the targeting experiments, the assistance system was fixed to a flexible holding arm (Fig.4) and needle insertion experiments were performed in a gelatin phantom containing fiducial targets (target number:12, mean diameter:7.6mm). For each target, real-time navigation was used with the POCC sequence (TR/TE=3.4/1.5ms,BW=898Hz/px,SLbSSFP=4.5mm,SLFLASH=10mm,FOV=243×300mm,matrix=192x156,TACycle=1.4s) until the projected needle pathway was aligned with the target center. Afterwards, the needle (ITP, Bochum, Germany) was inserted through the needle guide using the POCC sequence (Fig.2). For each target, the distance of the insertion point to the geometric center was assessed in a high-resolution (0.5mm³) 3D GRE data set (Fig.5).
Results
The piezo motors did not cause visible signal inferences during device operation at 1.5T (Fig.3). In the targeting experiments, all fiducials were successfully targeted using the tracking sequence in a mean total procedure time (instrument positioning + needle insertion) of 4.3±1.2min. For a target diameter of 7.6±0.5mm, a mean lateral distance of the insertion point to the target centers of 1.3±0.8mm and a longitudinal distance along the needle pathway of 1.8±0.8mm was found.Discussion & Conclusion
Handling of the device with the CU was intuitive. The achieved targeting accuracy is comparable to previous studies with the mechanical version of the assistance system4. Future work will focus on a proper safety evaluation and the application in more realistic clinical scenarios. With its flexible design, the device is not limited to percutaneous interventions but could for example be equipped with an ultrasound applicator. Based on this preliminary work, the system demonstrates its potential as an accurate and versatile tool with the potential to decrease procedure times and thus increase the clinical acceptance of MR-guided interventions.Acknowledgements
This work was supported in parts by a grant from the German Federal Ministry for Economic Affairs and Energy (BMWi) under the grant program “Zentrales Innovationsprogramm Mittelstand (ZIM),” grant number ZF4535603BA9, as part of the IraSME funding “E‐GantryMate.”References
1. Heerink, W. J. et al. Robotic versus Freehand Needle Positioning in CT-guided Ablation of Liver Tumors: A Randomized Controlled Trial. Radiology 181698 (2019) doi:10.1148/radiol.2018181698.
2. Busse, H., Kahn, T. & Moche, M. Techniques for Interventional MRI Guidance in Closed-Bore Systems. Top. Magn. Reson. Imaging 27, 9–18 (2018).
3. Arnolli, M. M., Hanumara, N. C., Franken, M., Brouwer, D. M. & Broeders, I. A. M. J. An overview of systems for CT- and MRI-guided percutaneous needle placement in the thorax and abdomen. Int. J. Med. Robot. 11, 458–475 (2015).
4. Reichert, A., Bock, M., Vogele, M. & Joachim Krafft, A. GantryMate: A Modular MR-Compatible Assistance System for MR-Guided Needle Interventions. Tomography 5, 266–273 (2019).
5. de Oliveira, A., Rauschenberg, J., Beyersdorff, D., Semmler, W. & Bock, M. Automatic passive tracking of an endorectal prostate biopsy device using phase-only cross-correlation. Magn. Reson. Med. 59, 1043–1050 (2008).
6. Krafft, A. J. et al. Passive marker tracking via phase-only cross correlation (POCC) for MR-guided needle interventions: Initial in vivo experience. Phys. Med. 29, 607–614 (2013).
7. Zamecnik, P. et al. Automated Real-time Needle-Guide Tracking for Fast 3-T MR-guided Transrectal Prostate Biopsy: A Feasibility Study. Radiology 273, 879–886 (2014).
8. Reichert, A. et al. Simultaneous slice excitation for accelerated passive marker tracking via phase-only cross correlation (POCC) in MR-guided needle interventions. Magn. Reson. Mater. Phys. Biol. Med. 31, 781–788 (2018).
9. Reichert, A., Reiss, S., Krafft, A. J. & Bock, M. Passive needle guide tracking with radial acquisition and phase-only cross-correlation. Magn. Reson. Med. 85, 1039–1046 (2021). 10. Reichert, A. et al. Passive Real-Time Needle Tracking and Needle Tip Estimation. in Proc. Intl. Soc. Mag. Reson. Med. vol. 30 (2022).
Figures