0747
Motorized template for MRI-guided focal cryoablation of prostate cancer1Brigham and Women's Hospital, Boston, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Physical Sciences Inc., Andover, MA, United States
Synopsis
Keywords: Interventional Devices, Prostate
MR-guided focal cryoablation of prostate cancer has often been selected as a minimally-invasive treatment option. Placing multiple cryo-needles accurately to form an ablation volume that adequately covers the target volume is crucial for a better oncological/functional outcome. This work presents an MRI-compatible system combining a motorized template grid and insertion depth sensing, which allows the physician to precisely place the cryo-needles into the desired location. Our preliminary results demonstrate the advantages of adding additional degrees of freedom to the template and providing the physician with real-time feedback on the insertion depth.Introduction
Focal treatment has often been selected as salvage treatment for post-radiation recurrences of prostate cancer (PCa) and, more recently, as a primary treatment option for low and intermediate-risk PCa patients1. Focal treatment aims to destroy only the localized diseased area and leave the rest of the organ intact through thermal ablations, such as high-intensity ultrasound and cryoablation2. MR-guided focal cryoablation (FC) offers advantages, such as visible frozen tissue boundaries, proven ability to treat post-radiation recurrence, and better access to anterior lesions. One of the major technical challenges for FC is to plan and deploy multiple cryo-needles accurately to form an ablation volume that adequately covers the target volume while sparing critical structure around it. The conventional needle-guiding grid template, typically with 5-mm hole intervals, limits the physician’s ability to fine-tune the needle locations or to choose different insertion paths. Several robotics devices for MRI-guided prostate biopsies have been published in the past two decades3-5. However, previously published devices were optimized for biopsy or brachytherapy, which requires one needle insertion at a time and are not suitable for FC, where multiple needles are placed simultaneously. To this date, no MRI-conditional device has been presented to accurately guide multiple cryo-needles in FC of PCa. In this study, we developed a new MRI-compatible motorized cryoablation template with a camera-based needle depth sensor, and tested if it improves the needle placement accuracy in a phantom.Methods
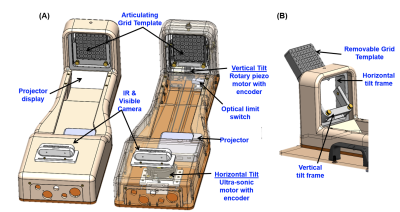
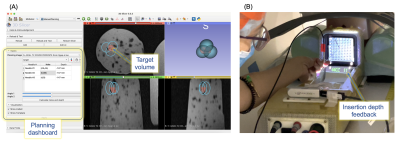
Motorized template: The device can angulate in two degrees of freedom a standard grid template with a 5 mm spacing distance between holes in the size of 60 x 60 mm (AccuCare Disposable Template Gird, CIVCO). The template is mounted on a 3D-printed resin gimbal and tightened with two brass knobs for easy insertion/removal. The angulation of the template grid is performed with an MR-compatible ultrasonic rotary motor (USR60-E3N, Shinsei Corp.) for horizontal angulation and a piezo rotary motor (LR-23-050, PiezoMotor) for vertical angulation (Fig. 1). A pneumatic footswitch controls the power provided to the motors, which are powered off while scanning. The device can articulate ±15° in the vertical and horizontal position, and multiple needles can be simultaneously inserted through the template grid at the defined angles. The device also has an MR-conditional depth camera (Realsense D435, Intel) to measure in real-time the insertion depth. A stereo vision algorithm uses the needle and template images to calculate the depth position of each needle in real time and display them as feedback for the user.User interface and feedback: The user interface was implemented on 3D Slicer6 and provides an interactive graphical environment for the user to 1) review pre- and intra-procedural MRI, 2) define target volume, 3) plan the cryo-needle placement and iceball orientation (Fig. 2A), and 4) control the device. Additionally, a portable projector (Luma150, Kodak) mounted on the device projects which hole to insert the needle and the current needle insertion depth on the template (Fig. 2B).
Experiments: (1) SNR Analysis. The impact of the device on the MR image quality was evaluated by measuring the signal-to-noise-ratios (SNR) of images acquired with and without the presence of the device in the bore. T2-weighted images were acquired using a turbo spin echo (TSE) sequence (TR/TE=4800/86ms, flip-angle=173, matrix=512x512, slice thickness=3mm, FOV=160x160mm) in a 3-Tesla MRI scanner (MAGNETOM Skyra 3T, Siemens Healthineers). (2) Placement Accuracy Evaluation. We also performed a study to assess the improvement of the targeting accuracy using the proposed device in an agar phantom. We conducted ten paired insertions toward randomly located targets. We divided the insertions into two groups: 1) five straight insertions without depth measurement feedback (Group A) and 2) five insertions using all device capabilities (Group B). Targeting error was defined as the 3D Euclidean distance between desired and achieved target locations. The needle tip location was manually measured on post-insertion T2w MR images.
Results
SNR Analysis: The SNRs with and without the presence of the device were 20.4 and 11.6 respectively, and are in line with our previous work7.Placement Accuracy Evaluation: The straight insertions without depth feedback resulted in a targeting error of 13.3±5.5mm, while the angulated insertions with depth measurement feedback had a targeting error of 5.0±1.9mm (p < 0.05).
Discussion
The results suggest that including additional degrees of freedom in the template orientation and the insertion depth feedback can improve the accuracy of cryo-needle placement. The average placement error was reduced by 62% when using the device angulation and depth feedback. Manually placing the needle at the corrected insertion depth is a challenging task and was the most significant source of error in Group A insertions. Different from prostate biopsies, placing the needles at proper insertion depth is crucial in focal cryoablation procedures, affecting not only the final ablation volume but also increasing the risk of damage to surrounding structures.Conclusion
This work demonstrates that the combination of the motorized template with the insertion depth feedback has the potential to improve the cryo-needle placement accuracy. The system will be integrated with a data-driven planning algorithm to guarantee a proper ablation margin while preserving critical structures.Acknowledgements
The study was funded in part by the National Institutes of Health (R43EB032676, R01EB020667, R01CA235134 and P41EB028741)References
[1] van Luijtelaar A, Fütterer JJ, Bomers JG. Minimally invasive magnetic resonance image-guided prostate interventions. Br J Radiol. 2022;95(1131):20210698.
[2] Tourinho-Barbosa RR, Sanchez-Salas R, Claros OR, et al. Focal Therapy for Localized Prostate Cancer with Either High Intensity Focused Ultrasound or Cryoablation: A Single Institution Experience. J Urol. 2020;203(2):320-330.
[3] angos S, Eichler K, Engelmann K, et al. MR-guided transgluteal biopsies with an open low-field system in patients with clinically suspected prostate cancer: technique and preliminary results. Eur Radiol. 2005;15(1):174-182.
[4] Moreira P, Patel N, Wartenberg M, et al. Evaluation of robot-assisted MRI-guided prostate biopsy: needle path analysis during clinical trials. Phys Med Biol. 2018;63(20):20NT02.
[5] Stoianovici D, Kim C, Srimathveeravalli G, et al. MRI-Safe Robot for Endorectal Prostate Biopsy. IEEE ASME Trans Mechatron. 2013;19(4):1289-1299.
[6] Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30(9):1323-1341.
[7] Moreira P, Grimble J, Iftimia N, et al. In vivo evaluation of angulated needle-guide template for MRI-guided transperineal prostate biopsy. Med Phys. 2021;48(5):2553-2565.
Figures