0744
A flat fully-populated MR-guided focused ultrasound phased array body system1Sunnybrook Research Institute, Toronto, ON, Canada, 2Arrayus Technologies Inc., Burlington, ON, Canada, 3Sunnybrook Health Sciences Centre, Toronto, ON, Canada, 4University of Toronto, Toronto, ON, Canada
Synopsis
Keywords: Uterus, MR-Guided Interventions
Existing commercial body MR-guided focused ultrasound (MRgFUS) systems have limited electronic steering ranges and rely on mechanical translation/rotation to ablate large tissue volumes. Here, we describe a flat fully-populated MRgFUS phased array body system with increased electronic steering capabilities. Following extensive benchtop and pre-clinical testing, the device was evaluated in a pilot clinical trial of MRgFUS for the treatment of uterine fibroids, which demonstrated the feasibilty and safety of the approach. The technology is extensible, stackable, and modular, allowing custom MRgFUS device development tailored to any indication.
Introduction
MR-guided focused ultrasound (MRgFUS) for non-invasive thermal surgery has been demonstrated in various clinical applications1-3. Phased array FUS applicators provide electronic control over the applied acoustic beam geometry, and can be tailored to provide optimal energy delivery patterns for specific therapeutic indications. Existing commercial body MRgFUS systems employ large-element spherically-curved arrays with limited electronic steering ranges, and therefore rely on mechanical translation/rotation to treat large tissue volumes1,4. Here, we describe a flat fully-populated MRgFUS phased array system for body applications with increased electronic steering capabilities. The feasibility of using this novel design for image-guided thermoablation over large target volumes was demonstrated in pre-clinical and clinical studies.Methods
Lead zirconate titanate (PZT) transducer elements were grouped into 8 x 8 square modules with a half-wavelength inter-element spacing at the driving frequency (f ≈ 0.5 MHz, lateral vibration mode)5-7. A flat 170 mm-diameter 96-module (6144-element) array was fabricated in house (Fig. 1). Custom driving electronics were developed using Application-Specific Integrated Circuits (ASICs), enabling element-wise phase and amplitude control over the array aperture. The MRI-compatible transducer array and driving system were designed to mount on the standard bed of a 3T MRI scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). Online 3D visualization of the estimated in-situ acoustic pressure field distribution was integrated within the driving system software’s graphical user interface (GUI) for treatment planning. A sparse 64-element boundary receiver array was integrated for cavitation monitoring.The device underwent extensive pre-clinical testing, and was evaluated in a pilot clinical trial of MRgFUS for the treatment of uterine fibroids (NCT03323905). T2-weighted MRI scans (3D SPACE turbo spin echo; TR: 2000 ms, TE: 81 ms, slice thickness: 2 mm) were acquired for targeting and delineation of “no-pass” zones within the ultrasound beam path (e.g., scar tissue, air bubbles, nerves, bowel, catheter tip in bladder). Multi-planar proton resonance frequency (PRF) shift MR-thermometry (FLASH gradient-echo; TR: 48 ms, TE: 20 ms, in-plane resolution: 2 mm x 2 mm, slice thickness: 3 mm, two planes every 6.1 s) and thermal dose mapping were carried out for intraoperative treatment monitoring. Contrast-enhanced T1-weighted (CE-T1w) MRI scans (VIBE 3D gradient-echo; TR: 3.4 ms, TE: 1.3 ms, slice thickness: 3 mm) were acquired to map the ablation zones post-treatment. Patients were followed for 12 months post-treatment (clinical/MR imaging, symptom surveys).
Results
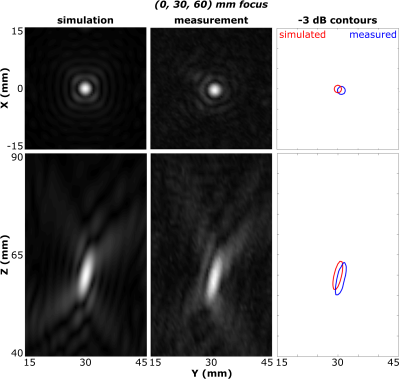
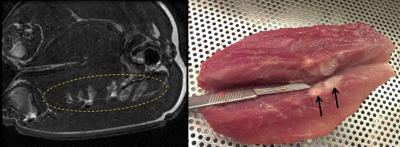
Acoustic Characterization: Hydrophone measurements of the acoustic field generated by the phased array demonstrated ellipsoidal focal volumes; at a target depth of 6 cm the focal beam width and depth of field (pressure full width at half maximum) were 2.7 mm and 11.0 mm, consistent with numerical simulations8 (Fig. 2). The system was shown capable of rapid electronic beam steering at up to 250 treatment points/second, with the targets distributed arbitrarily across the array aperture.Pre-Clinical Testing: FUS exposures were performed in in-vivo porcine muscle tissue (Fig. 3). Over 860 sonications targeting ablation were carried out across 29 animals. Volumetric tissue ablation was demonstrated via both single- and multi-point exposures, and confirmed via gross pathology and histology.
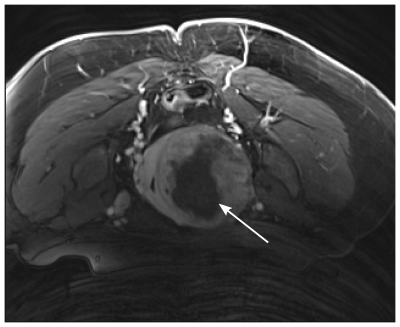
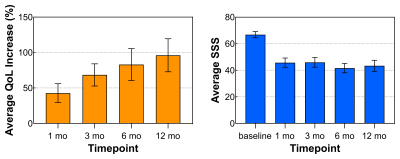
Clinical Trial: Patients (n = 50) received 24 ± 9 sonications (power = 151 ± 71 W, duration = 43 ± 11 s) during MRgFUS treatment sessions (74 ± 26 [145 ± 28] min FUS [MRI] time) resulting in thermal dose volumes of 25 ± 20 cm3 (240 cumulative equivalent minutes at 43oC). Active cooling and the low operating frequency permitted sequential sonications in quick succession with minimal near-field heating, shortening treatment times. The mean nonperfused tissue volume measured immediately post-treatment via CE-T1w MRI was 80 ± 130 cm3 (example image provided in Fig. 4). Increases observed in quality of life (QoL) scores improved throughout the year post-treatment, and symptom severity scores (SSS) improved 1 month post-treatment and were durable throughout the year (Fig. 5). There were no serious adverse events (e.g., off-target effects, near-field damage) related to the use of the MRgFUS device.
Discussion
Volumetric MRgFUS thermoablation with a flat fully-populated phased array appears safe and effective for the treatment of uterine fibroids. The MRgFUS device is capable of large volume tissue ablation without the need for mechanical translation, which simplifies system design and enables precise MR-thermometry without motion-induced artifacts. The system’s high element count provides increased control over the beam geometry for improved ultrasound energy delivery. The technology is extensible, stackable, and modular, allowing custom MRgFUS device development tailored to any indication.Acknowledgements
The authors thank A. Minhas, S. Philip, H. Meirovich, M. Kazem, R. Ramdoyal, P. Wu, R. Endre, and D. DiTommaso for technical assistance. Financial support for this work was provided by a Federal Development Grant, the Ontario Institute of Cancer Research, the Ontario Research Fund and Arrayus Technologies.References
1. Tempany CMC et al. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology. 2003;226(3):897-905.
2. Napoli A et al. Osteoid osteoma: MR-guided focused ultrasound for entirely noninvasive treatment. Radiology. 2013;267(2):514-21.
3. Elias WJ et al. A pilot study of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med. 2013;369(7):640-8.
4. Köhler MO et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med. Phys. 2009;36(8):3521-35.
5. Song J et al. Large improvement of the electrical impedance of imaging and high-intensity focused ultrasound (HIFU) phased arrays using multilayered piezoelectric ceramics coupled in lateral mode. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2012;59(7):1584-95.
6. Ellens NPK et al. A novel, flat, electronically-steered phased array transducer for tissue ablation: preliminary results. Phys. Med. Biol. 2015;60(6):2195-215.
7. Aslani P et al. Thermal therapy with a fully electronically steerable HIFU phased array using ultrasound guidance and local harmonic motion monitoring. IEEE Trans. Biomed. Eng. 2020;67(7):1854-62.
8. Ellens N et al. The utility of sparse 2D fully electronically steerable focused ultrasound phased arrays for thermal surgery: a simulation study. Phys. Med. Biol. 2011;56(15):4913-32.
Figures