0737
Skeletal muscle Mg2+, membrane permeability, and pH are altered in Becker muscular dystrophy: A 31P-MRS and DT-MRI study1C.J. Gorter MRI Center, Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 2Department of Radiology and Nuclear Medicine, Amsterdam UMC, Location AMC, Amsterdam, Netherlands, 3Department of Neurology, Leiden University Medical Center, Leiden, Netherlands
Synopsis
Keywords: Muscle, Permeability, Neuromuscular diseases
Becker muscular dystrophy (BMD) is an X-linked disorder characterised by variable, progressive muscle damage and loss of function. MR-derived fat fraction is used as a disease-progression marker, but early markers are lacking. Here we compared membrane permeability measures between BMD patients and controls to test whether these are altered in BMD. Phosphorus-(31P)-MRS showed reduced ionised magnesium [Mg2+], and increased phosphodiester/adenosine-triphosphate ratios and pH in the tibialis anterior muscle in patients, while diffusion-tensor-(DT)-MRI-derived permeability showed no inter-group differences. Future studies should determine the predictive value of 31P-MRS-measured [Mg2+], pH, and membrane breakdown for disease progression, to establish their potential as biomarkers.Introduction
Becker muscular dystrophy (BMD) is an X-linked neuromuscular disorder caused by mutations in the dystrophin gene. It is characterised by progressive muscle wasting and weakness, with hallmarks including muscle membrane instability and fat replacement. The latter reflects disease progression, which is considered irreversible and can be assessed using fat-water MRI as a biomarker. For interventions to be administered prior to loss of muscle function, imaging-based treatment monitoring biomarkers are needed that focus on disease activity. For instance, in Duchenne muscular dystrophy (DMD), a more severe form of BMD, ionic homeostasis is known to be disturbed1, leading to reduced intracellular ionised magnesium (Mg2+) as measured by phosphorus-(31P)-MRS.2 This is likely due to membrane leakiness, which itself reflects disease activity. Magnesium therefore merits assessment as a biomarker in BMD, alongside other potential disease activity metrics such as 31P-MRS phosphodiesters (PDE)—which reflect membrane phospholipid breakdown and are known to be elevated in BMD3—and the random permeable barrier model (RPBM)4,5, which measures muscle permeability and fibre diameter using multi-diffusion-time diffusion-tensor-(DT)-MRI data.In this study, we compared intramuscular [Mg2+], 31P-MRS metrics, and RPBM membrane permeability between adult BMD patients and healthy controls and assessed their correlations.
Methods
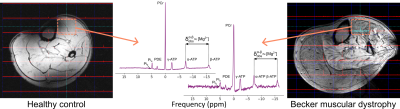
From our larger study cohort3, we included 13 BMD patients—age 20-59yrs—and 9 male controls—age 23-65yrs—who underwent both 7T 31P-MRS and 3T multi-diffusion-time DT-MRI (Achieva and Ingenia systems, respectively; Philips, Best, NL). At 7T, data were obtained in the left lower-leg with a dual-tuned 1H/31P volume coil.3 At 3T, colocalised images were acquired with a 16-element torso array and 12-element posterior array for reception.At 7T, T1-weighted anatomical acquisitions were followed by second-order image-based shimming, then 2D 31P chemical shift imaging with: TR/TE=2,000/0.5ms, field-of-view=160×200mm, matrix size=8×10, 2048 complex data points, bandwidth=4,000Hz, block excitation with FA=45°, and acquisition weighting with 24 NSA at the centre of k-space. At 3T, chemical-shift-based fat-water gradient-recalled-echo MRI was followed by spin-echo-(SE)- and stimulated-echo-(STE)-DT-MRI with: TR/TE=5,000/58ms; field-of-view=384×384mm; matrix size=96×96; 9 slices, 6mm thickness, 3mm gap; b-values=0 and 400s/mm2; 12 directions; SENSE factor=1.7; diffusion times, Δ=27,130,330ms; and comprehensive fat suppression.6
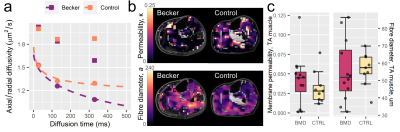
The 31P spectra were Hamming-filtered, then visualised in 3DiCSI (v1.911). Free-induction decays were output for the tibialis anterior (TA) muscle, for future histology comparisons, and analysed using a Python pipeline7, where metabolite ratios, weighted pH8, and [Mg2+] were calculated. DT-MRI data were pre-processed in MATLAB (2019a).6 TA ROIs were drawn on fat-water images in MIPAV, and radial diffusivities per diffusion time were fitted with the RPBM (https://github.com/NYU-DiffusionMRI/RPBM), producing membrane permeabilities and fibre diameters. Voxels with SNR < 20 or fat-fraction > 80% were excluded.
Statistical analysis was performed in R (v4.1). Groups were compared via t-tests if normally distributed, or Mann-Whitney U-tests if not. Correlations were assessed via Pearson’s r, and p<0.05 was considered statistically significant.
Results
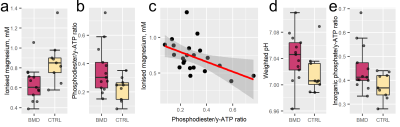
Representative 31P-MRS and DT-MRI data are shown in Figs 1 and 2, respectively, with 31P-MRS results in Fig. 3. The 31P-MRS-derived [Mg2+] in the TA was lower in BMD versus controls: mean (SD)=0.63 (0.17) vs 0.86 (0.22)mM, respectively; p=0.022. PDE/γ-ATP was larger in patients versus controls: mean (SD)=0.36 (0.17) vs 0.22 (0.09), respectively; p=0.029. Comparing both metrics, [Mg2+] decreased with increasing PDE/γ-ATP: Pearson’s r=–0.49, p=0.002. Weighted pH was more alkaline in patients: median (IQR) = 7.04 (0.04) vs 7.01 (0.03), respectively. Pi/ATP did not differ.RPBM-DT-MRI showed similar permeabilities in patients versus controls—0.042 (0.033) vs 0.032 (0.021)μm/ms, respectively, p=0.410—though markedly high- and low-permeability outliers were evident in both groups. RPBM permeabilities did not correlate with [Mg2+] or PDE/γ-ATP (p=0.441, 0.874, respectively), but RPBM fibre diameters were larger in patients, as we reported previously.9
Discussion
In this 31P-MRS and DT-MRI study of membrane permeability in BMD, we show reduced [Mg2+] and increased PDE/γ-ATP and weighted pH in BMD patients versus controls, suggesting value as biomarkers for membrane leakiness. RPBM membrane permeability, however, showed no differences.Our magnesium results agree with work in DMD2, where [Mg2+] was reduced in patients as compared to controls. This has functional consequences, as magnesium is implicated in muscle contraction and mitochondrial function. We also observed an elevated pH in BMD patients versus controls. Reyngoudt et al. showed two subgroups of DMD patients with reduced [Mg2+]: one with more-alkaline 1H-MRS-derived intracellular pH, similar to our patient group, and another where pH was unchanged.2 Similarly, we, and others, showed increased PDE/γ-ATP in DMD patients versus controls2,10,11, and previously showed increased PDE/γ-ATP in our BMD cohort but no change in pH calculated using a single inorganic phosphate peak3,12, as opposed to our two-peak weighted pH.8
We observed no group differences in RPBM-derived membrane permeabilities, similar to work in the same cohort using DT-MRI mean diffusivity3—a less-specific permeability marker. Further, no correlations were observed with PDE/γ-ATP or [Mg2+], perhaps because these metrics reflect different aspects of membrane integrity, or because fibrosis is not modelled in the RPBM.
Conclusions
We show reduced intramuscular [Mg2+] and increased PDEs and weighted pH in BMD patients versus controls, suggesting greater membrane permeability and possible applications as biomarkers of disease activity, while DT-MRI-measured permeability did not differ. Further work will examine [Mg2+] in our larger patient cohort, in all lower-leg muscles, with comparisons to TA-muscle histology, functional measures, and fat fraction.Acknowledgements
We are grateful to Harmen Reyngoudt and Chloé Najac for their helpful insights.References
1. Allen DG, Whitehead NP. Duchenne muscular dystrophy–what causes the increased membrane permeability in skeletal muscle? The International Journal of Biochemistry & Cell Biology. 2011; 43(3):290-4.
2. Reyngoudt, H, Lopez Kolkovsky, AL, Carlier, PG. Free intramuscular Mg2+ concentration calculated using both 31P and 1H NMRS-based pH in the skeletal muscle of Duchenne muscular dystrophy patients. NMR in Biomedicine. 2019; 32:e4115.https://doi.org/10.1002/nbm.4115
3. Hooijmans, MT, Froeling, M, Koeks, Z, et al. Multi-parametric MR in Becker muscular dystrophy patients. NMR in Biomedicine. 2020; 33:e4385. https://doi.org/10.1002/nbm.4385
4. Novikov DS, Fieremans E, Jensen JH, Helpern JA. Random walks with barriers. Nature Physics. 2011;7(6):508-14.
5. Sigmund EE, Novikov DS, Sui D, Ukpebor O, Baete S, Babb JS, Liu K, Feiweier T, Kwon J, McGorty K, Bencardino J. Time‐dependent diffusion in skeletal muscle with the random permeable barrier model (RPBM): application to normal controls and chronic exertional compartment syndrome patients. NMR in Biomedicine. 2014; 27(5):519-28.
6. Burakiewicz J, Hooijmans MT, Webb AG, Verschuuren JJ, Niks EH, Kan HE. Improved olefinic fat suppression in skeletal muscle DTI using a magnitude‐based Dixon method. Magnetic Resonance in Medicine. 2018; 79(1):152-9.
7. Cameron D, Welch AA, Adelnia F, Bergeron CM, Reiter DA, Dominguez LJ, Brennan NA, Fishbein KW, Spencer RG and Ferrucci L (2019) Age and muscle function are more closely associated with intracellular magnesium, as assessed by 31P magnetic resonance spectroscopy, than with serum magnesium. Front. Physiol. 10:1454. https://doi.org/10.3389/fphys.2019.01454
8. Reyngoudt, H, Turk, S, Carlier, PG. 1H NMRS of carnosine combined with 31P NMRS to better characterize skeletal muscle pH dysregulation in Duchenne muscular dystrophy. NMR in Biomedicine. 2018; 31:e3839. https://doi.org/10.1002/nbm.3839
9. Cameron D, Burakiewicz J, van de Velde NM, Baligand C, Veeger TT, Hooijmans MT, Verschuuren JJ, Niks EH, Kan H. DT-MRI with the random permeable barrier model shows larger, more heterogeneous muscle fibre diameters in Becker muscular dystrophy patients. Proceedings of the International Society for Magnetic Resonance in Medicine, 29, 2021; 3173.
10. Hooijmans MT, Doorenweerd N, Baligand C, Verschuuren JJ, Ronen I, Niks EH, Webb AG, Kan HE. Spatially localized phosphorous metabolism of skeletal muscle in Duchenne muscular dystrophy patients: 24–month follow-up. PLoS One. 2017; 12(8):e0182086.
11. Hooijmans MT, Niks EH, Burakiewicz J, Verschuuren JJ, Webb AG, Kan HE. Elevated phosphodiester and T2 levels can be measured in the absence of fat infiltration in Duchenne muscular dystrophy patients. NMR in Biomedicine. 2017; 30(1):e3667.
12. Wokke BH, Hooijmans MT, van den Bergen JC, Webb AG, Verschuuren JJ, Kan HE. Muscle MRS detects elevated PDE/ATP ratios prior to fatty infiltration in Becker muscular dystrophy. NMR in Biomedicine. 2014; 27(11):1371-7.
Figures