0735
MR elastography-based slip interface imaging (SII) to assess the mobility of the myofascial interface in extremities: A feasibility study1Radiology, Mayo Clinic, Rochester, MN, United States, 2Physiology and Biomedical Engineering, Mayo Clinic, Rochester, MN, United States, 3General Internal Medicine, Mayo Clinic, Rochester, MN, United States
Synopsis
Keywords: Muscle, Elastography, slip interface imaging, myofascial interface, myofascial pain syndrome
Myofascial pain syndrome (MPS) is a common chronic pain disorder that can cause disability. Efforts to understand the MPS pathology have focused on myofascial connective tissue and the function of fascial plane mobility. Slip interface imaging (SII) offers a unique opportunity to assess fascial plane mobility noninvasively. Here, we investigated the feasibility of SII to visualize the mobility of the intermuscular myofascial interface in the upper leg and the functional intramuscular interface in the forearm flexor muscles in healthy volunteers. This creates a foundation for using MRE/SII to distinguish between a healthy and a dysfunctional fascial plane in MPS patients.Introduction
Myofascial pain syndrome (MPS) is a common public health problem. It is a pain condition originating from muscle and surrounding fascia that can affect many skeletal muscles.1 The underlying mechanisms for MPS continue to be investigated, with a greater focus on impairments involving myofascial connective tissue and the function of fascial plane mobility.2,3 Development of technology capable of providing biomarkers that noninvasively characterize the mobility of the fascial plane would contribute significantly to the diagnosis and assessment of therapeutic modalities. Previous ultrasound studies have evaluated the sliding capability of thoracolumbar fascial layers and found significant correlations between fascial plane mobility and trunk flexion/ extension range of motion in men with chronic low back pain.4 Compared to ultrasound, MR elastography (MRE) can offer a unique opportunity to assess fascial plane mobility given its high sensitivity to very small motions, a FOV unencumbered by acoustic window requirements, and the ability to obtain full 3D displacement throughout a 3D volume. In muscle MRE, mechanical vibrations are introduced into muscles via specific MRE drivers that couple to the muscle groups of interest.5-8 With the encoded motion, a few attempts have been made to view functional shear interfaces in volunteer studies of forearms.9,10 Slip interface imaging (SII), a recently developed MRE-based technique, has used shear strain mapping to quantify the adherence between two adjacent tissue layers.11-13 In this study, we propose to adapt SII to assess the mobilities of the myofascial interface in the upper leg and the forearm in healthy volunteers.Methods
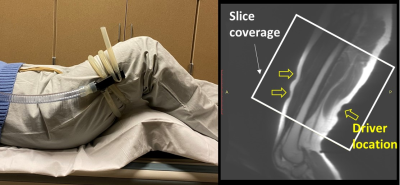
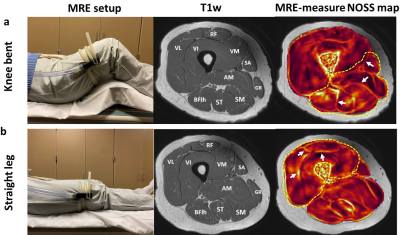
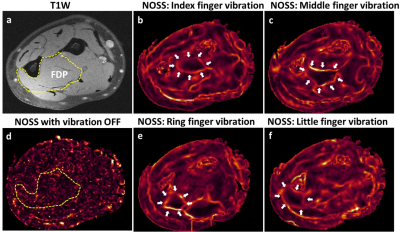
Three healthy volunteers were scanned on a 3T MR scanner using a dual-sensitivity SE-EPI-MRE pulse sequence.14 For upper leg MRE, vibrations were introduced with a pneumatic tube passive driver wrapped and clamped around the subject’s thigh (Fig.1). Two MRE scans were separately performed with the volunteers in a supine position with the knees bent or straight to evaluate the effects of different muscular states on the intermuscular myofascial slip interface (40 Hz vibration, TR/TE=5400/48ms, 72 oblique axial slices, 3mm slice thickness, 3 phase offsets). For forearm MRE, selective vibration of the individual finger (index, middle, ring, and little) was performed via a small drum-like pneumatic finger driver, by which the motion was transferred through the structurally connected tendons to the forearm flexor muscles (Fig.2). This setup was used to evaluate the functional slip interface of the flexor digitorum profundus (FDP). The volunteers were in a prone position with either the right or left hand extended above the head, palm facing down with the selected finger attached to the driver by double-stick tape (90 Hz vibration, TR/TE=1400/42ms, 18 oblique axial slices, 4mm slice thickness, 4 phase offsets). Normalized octahedral shear strain (NOSS) maps were calculated from the measured displacement fields as previously described.12Results and Discussion
In response to an external shear force, the fascial layers that surround and separate muscles can deform or slide relative to each other, depending on the fascial plane's mobility. A restriction-free interface would slip and cause shear displacement discontinuity (i.e., large shear strain) across the interface, while a restricted interface that experiences a shear force will exhibit displacement continuity (i.e., small shear strain). As shown in Fig.3, the slip interface can be observed as large NOSS values at the intermuscular myofascial interfaces, indicating normal sliding and gliding capability of the fascial planes. Distinct slip interface patterns were observed at two different muscular states: the myofascial slip interfaces are well-depicted between posterior and medial muscle compartments but not on the anterior quadriceps with the bent-knee position. Conversely, the trend is the opposite when the leg is straight. With the knee bent, the gentle stretch of the anterior part of the leg may tighten the quadriceps, leading to restricted relative motion between VL, VI, and RF, whereas the hamstrings were relaxed, and all corresponding intramuscular fascia planes can move without restriction. Then with the straight leg, the quadriceps were relaxed, and the hamstrings were in a gently stretched state. The differences between the two muscular states were sensitively depicted by the shear strain (i.e., NOSS) occurring at the fascial interface.Furthermore, SII can visualize the functional slip interface within a muscle that does not have a clear anatomical boundary. Fig.4 shows the NOSS maps where individual fingers controlled by the FDP were vibrated independently. It is known that individuated finger movements are due to the selective activation of functional compartments within each muscle specific to each finger.15 NOSS maps demonstrated that there exist spatially distinct and localized functional slip interfaces within the FDP for different fingers, demonstrating the functional slip interfaces of the FDP. In comparison, a high NOSS contour is not seen with no externally applied motion.
Conclusion
This study demonstrated the feasibility of utilizing MRE-based SII to assess the mobility of the intermuscular myofascial interface in the upper leg and the functional intramuscular interface in the forearm flexor muscles. This would create a foundation for using MRE/SII to distinguish between a healthy fascial plane with a slip boundary and a dysfunctional, adhesive fascial plane in MPS patients. The results provide strong motivation for a more extensive investigation of this unique quantitative approach for assessing myofascial pain syndrome and response to therapeutic measures.Acknowledgements
This work was supported by grants from the NIH (R01 EB001981, R61 AT01218, and R01 NS113760).References
1. Gerber, L. H. et al. A systematic comparison between subjects with no pain and pain associated with active myofascial trigger points. PM & R : the journal of injury, function, and rehabilitation 5, 931-938, doi:10.1016/j.pmrj.2013.06.006 (2013).
2. Stecco, A., Gesi, M., Stecco, C. & Stern, R. Fascial components of the myofascial pain syndrome. Curr Pain Headache Rep 17, 352, doi:10.1007/s11916-013-0352-9 (2013).
3. Langevin, H. M. Fascia Mobility, Proprioception, and Myofascial Pain. Life (Basel) 11, 668, doi:10.3390/life11070668 (2021).
4. Langevin, H. M. et al. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet Disord 12, 203-203, doi:10.1186/1471-2474-12-203 (2011).
5. Basford, J. R. et al. Evaluation of healthy and diseased muscle with magnetic resonance elastography. Archives of Physical Medicine and Rehabilitation 83, 1530-1536, doi:https://doi.org/10.1053/apmr.2002.35472 (2002).
6. Bensamoun, S. F. et al. Determination of thigh muscle stiffness using magnetic resonance elastography. Journal of Magnetic Resonance Imaging 23, 242-247, doi:https://doi.org/10.1002/jmri.20487 (2006).
7. Chakouch, M. K., Charleux, F. & Bensamoun, S. F. Quantifying the Elastic Property of Nine Thigh Muscles Using Magnetic Resonance Elastography. PLoS One 10, e0138873, doi:10.1371/journal.pone.0138873 (2015).
8. Creze, M. et al. Magnetic resonance elastography of the lumbar back muscles: A preliminary study. Clinical Anatomy 31, 514-520, doi:https://doi.org/10.1002/ca.23065 (2018).
9. Mariappan, Y. K., Glaser, K. J., Manduca, A. & Ehman, R. L. Cyclic motion encoding for enhanced MR visualization of slip interfaces. J Magn Reson Imaging 30, 855-863, doi:10.1002/jmri.21914 (2009).
10. Mariappan, Y. K. et al. Vibration imaging for localization of functional compartments of the extrinsic flexor muscles of the hand. J Magn Reson Imaging 31, 1395-1401, doi:10.1002/jmri.22183 (2010).
11. Yin, Z. et al. Slip interface imaging predicts tumor-brain adhesion in vestibular schwannomas. Radiology 277, 507-517, doi:10.1148/radiol.2015151075 (2015).
12. Yin, Z. et al. Slip interface imaging based on MR-elastography preoperatively predicts meningioma–brain adhesion. J Magn Reson Imaging 46, 1007-1016, doi:10.1002/jmri.25623 (2017).
13. Yin, Z. et al. A new method for quantification and 3D visualization of brain tumor adhesion using slip interface imaging in patients with meningiomas. European Radiology 31, 5554-5564, doi:10.1007/s00330-021-07918-6 (2021).
14. Yin, Z. et al. In vivo characterization of 3D skull and brain motion during dynamic head vibration using magnetic resonance elastography. Magn Reson Med 0, doi:10.1002/mrm.27347 (2018).
15. Fleckenstein, J. L., Watumull, D., Bertocci, L. A., Parkey, R. W. & Peshock, R. M. Finger-specific flexor recruitment in humans: depiction by exercise-enhanced MRI. J Appl Physiol (1985) 72, 1974-1977, doi:10.1152/jappl.1992.72.5.1974 (1992).
Figures