0721
Quantitative DCE-MRI after P4-based error correction provides a more accurate therapy response assessment of pancreatic ductal adenocarcinoma1Interdisciplinary Engineering, University of Alabama at Birmingham, Birmingham, AL, United States, 2Biomedical Engineering, University of Alabama at Birmingham, Birmingham, AL, United States, 3Medicine, University of Alabama at Birmingham, Birmingham, AL, United States, 4Surgery, University of Alabama at Birmingham, Birmingham, AL, United States, 5Radiology, University of Alabama at Birmingham, Birmingham, AL, United States, 6Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States, 7Medicine, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Keywords: Pancreas, Cancer, DCE-MRI, Pancreatic cancer, Therapy monitoring, Perfusion phantom, Quantification
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) measures tissue perfusion by monitoring the dynamic change of MRI contrast agents. However, the inter/intra-scanner variability in quantitative DCE-MRI (qDCE) measurement remains a concern. We developed a point-of-care portable perfusion phantom (P4) that can be imaged with a human subject in a standard MRI scanner to detect and correct the inter/intra-scanner variability of qDCE measurement. We demonstrated that the PDAC response to chemotherapy could be accurately assessed using quantitative DCE-MRI after our P4-based error correction method approximately seven weeks after starting therapy in two clinics.Introduction
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) measures tissue perfusion by monitoring the dynamic change of MRI contrast agents1-4. However, the primary barrier to the widespread clinical use of this technique is the inter/intra-scanner variability in quantitative DCE-MRI (qDCE) measurement5. To address this concern, we developed a point-of-care portable perfusion phantom (P4)6, 7. The P4 phantom can be imaged with a human subject in a standard MRI scanner to detect and correct the inter/intra-scanner variability of qDCE measurement6, 7. This study aimed to determine whether qDCE could assess pancreatic ductal adenocarcinoma (PDAC) response to chemotherapy early and accurately when the P4 phantom is employed to correct MRI scanner-driven errors.Method
DCE-MRI was applied to PDAC patients together with P4 phantoms before and approximately seven weeks after starting chemotherapy in one of three 3T MRI scanners of two institutes (GE Signa and SIEMENS Prisma at the University of Alabama at Birmingham (UAB); Philips Elition at Vanderbilt University Medical Center (VUMC)). Two patients had resectable PDAC, five patients had borderline resectable PDAC, seven had locally advanced PDAC, and two had metastatic liver lesions at diagnosis. All subjects entered systemic chemotherapy with FOLFIRINOX (n=14), Abraxane+Gemcitabine (n=1), or Abraxane+Cisplatin (n=1). Volume transfer constant (Ktrans), a measure of micro-perfusion, was calculated in the tumor region based on the extended Tofts model (ETM) before and after P4-based error correction. ETM provided the highest reproducibility of Ktrans in the abdominal tissues in our previous study7. The response of primary PDAC was determined about 16 weeks after therapy initiation based on RECIST criteria. Completed/partial responses were considered responding, while stable/progressive diseases were considered non-responding to therapy. Among the tumors classified as stable diseases, if the tumor size decreased by more than 10%, we considered them favorably responded to the therapy as well. The Ktrans changes in the tumors that favorably responded to chemotherapy were statistically compared to those in non-responding tumors using ANOVA. All data are given as mean±SD.Results
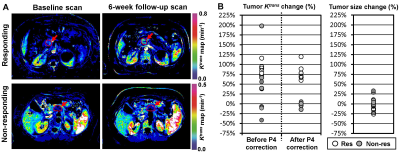
A total of 16 patients (5 female, 11 males; 4 Black, 12 White; age range = 46-78 years, median age = 64 years) were recruited. The mean primary tumor size in the baseline scan was 35±14 mm. Eight subjects responded favorably to chemotherapy, and the other eight did not. Figure 1A shows the ETM-based Ktrans maps of two representative patients responding and non-responding to chemotherapy. Figure 2A summarizes the tumor Ktrans and size changes during chemotherapy. The Ktrans after P4-based error correction increased 79±19% in the responding tumors, significantly higher than in the non-responding tumors (-4±8%, p<0.0001). However, before P4-based error correction, the Ktrans changes in the responding and non-responding tumors were 81±22% and 36±75%, respectively, without a statistical difference (p=0.1324). The size change of responding tumors in the first follow-up CT scans (i.e., 6-10 weeks after therapy initiation) was -14±8%, statistically different from non-responding tumors (10±14%, p=0.0008). However, the mean difference of Ktrans change between the responding and non-responding groups was about 3-fold higher than those of tumor size change when the P4 phantom was used for error correction.Conclusion and Discussion
We demonstrated that the PDAC response to chemotherapy was accurately assessed using quantitative DCE-MRI after our P4-based error correction method approximately seven weeks after starting therapy in two independent clinics. Our approach has the potential to help select therapy for PDAC patients with a greater likelihood of successful treatment. Early therapy response assessment will be imperative for patients with resectable (or borderline resectable) PDAC since it will enable the early determination of whether the neoadjuvant chemotherapy should be continued to facilitate margin-negative resection or if a change in systemic chemotherapy is warranted.Acknowledgements
This study was supported by the National Cancer Institute, UG3/UH3 CA232820, and the Radiology Research Pilot Award from the Department of Radiology at UAB.References
1. Barnes SL, Whisenant JG, Loveless ME, Yankeelov TE. Practical dynamic contrast enhanced MRI in small animal models of cancer: data acquisition, data analysis, and interpretation. Pharmaceutics. 2012;4(3):442-78. Epub 2012/10/30. doi: 10.3390/pharmaceutics4030442. PubMed PMID: 23105959; PMCID: 3480221.
2. Zhang CC, Yan Z, Giddabasappa A, Lappin PB, Painter CL, Zhang Q, Li G, Goodman J, Simmons B, Pascual B, Lee J, Levkoff T, Nichols T, Xie Z. Comparison of dynamic contrast-enhanced MR, ultrasound and optical imaging modalities to evaluate the antiangiogenic effect of PF-03084014 and sunitinib. Cancer medicine. 2014;3(3):462-71. Epub 2014/02/28. doi: 10.1002/cam4.215. PubMed PMID: 24573979; PMCID: 4101737.
3. Craciunescu OI, Blackwell KL, Jones EL, Macfall JR, Yu D, Vujaskovic Z, Wong TZ, Liotcheva V, Rosen EL, Prosnitz LR, Samulski TV, Dewhirst MW. DCE-MRI parameters have potential to predict response of locally advanced breast cancer patients to neoadjuvant chemotherapy and hyperthermia: a pilot study. Int J Hyperthermia. 2009;25(6):405-15. Epub 2009/08/07. doi: 10.1080/02656730903022700. PubMed PMID: 19657852; PMCID: 2783501.
4. Kim H, Folks KD, Guo L, Sellers JC, Fineberg NS, Stockard CR, Grizzle WE, Buchsbaum DJ, Morgan DE, George JF, Zinn KR. Early therapy evaluation of combined cetuximab and irinotecan in orthotopic pancreatic tumor xenografts by dynamic contrast-enhanced magnetic resonance imaging. Mol Imaging. 2011;10(3):153-67. Epub 2011/04/19. PubMed PMID: 21496446.
5. Kim H. Variability in Quantitative DCE-MRI: Sources and Solutions. J Nat Sci. 2018;4(1). Epub 2018/03/13. PubMed PMID: 29527572; PMCID: PMC5841165.
6. Kim H, Mousa M, Schexnailder P, Hergenrother R, Bolding M, Ntsikoussalabongui B, Thomas V, Morgan DE. Portable perfusion phantom for quantitative DCE-MRI of the abdomen. Med Phys. 2017;44(10):5198-209. Epub 2017/07/12. doi: 10.1002/mp.12466. PubMed PMID: 28692137; PMCID: PMC5646228.
7. Holland MD, Morales A, Simmons S, Smith B, Misko SR, Jiang X, Hormuth DA, Christenson C, Koomullil RP, Morgan DE, Li Y, Xu J, Yankeelov TE, Kim H. Disposable point-of-care portable perfusion phantom for quantitative DCE-MRI. Med Phys. 2022;49(1):271-81. Epub 20211210. doi: 10.1002/mp.15372. PubMed PMID: 34802148.
Figures