0720
Preoperative prediction of Ki-67 expression of hepatocellular carcinoma using T1 mapping on gadoxetic acid-enhanced MRI1Department of Radiology, Guangzhou First People’s Hospital, Guangzhou, China, 2Philips Healthcare, Guangzhou, China
Synopsis
Keywords: Liver, Cancer
The cell proliferation index, Ki-67 labeling index (LI) indicates the status of cell proliferation activity which corresponds with tumor biological behavior, treatment efficacy and prognosis. If the preoperative Ki-67 expression status in HCC can be accurately predicted noninvasively, it may provide important information for clinical decision-making. T1 mapping is useful for preoperative prediction of Ki-67 LI of HCC. The nomogram Combining T1 mapping on gadoxetic acid-enhanced MRI and clinical indicators has good predictive efficacy for preoperative prediction of Ki-67 LI, which can promote the individualized risk stratification and further treatment decision of HCC patients.Introdution
The cell proliferation index, Ki-67 labeling index (LI) indicates the status of cell proliferation activity which corresponds with tumor biological behavior, treatment efficacy and prognosis [1,2]. Previous studies have demonstrated that high Ki-67 LI was associated with poor overall survival, recurrence-free survival (RFS) [3-8]. Currently, the assessment of Ki-67 LI relies on postoperative immunohistochemical assays, and if preoperative Ki-67 LI of HCC can be accurately predicted noninvasively, it can provide important information for individualized treatment decision-making and interpretation of prognosis.Therefore, the aim of this study was to investigate the value of T1 mapping on gadoxetic acid-enhanced MRI in predicting Ki-67 LI in HCC patients preoperatively. Early recurrences after curative hepatectomy were also explored and compared in HCC patients with different Ki-67 LI.
Materials and methods
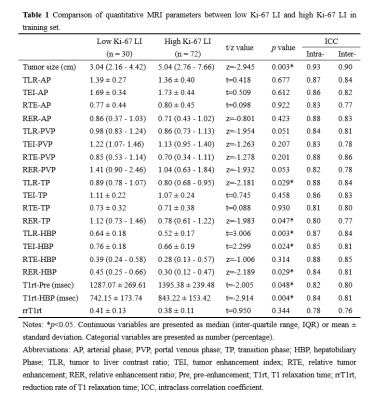
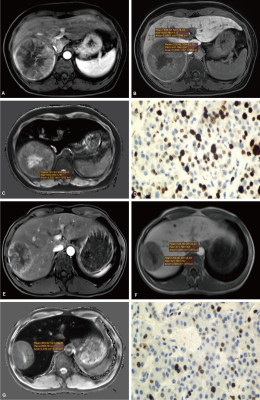
This retrospective study included 158 patients with surgically proven HCC who underwent preoperative T1 mapping on gadoxetic acid-enhanced MRI from two institutions. Patients from institution I (n = 102) and institution II (n = 56) were assigned to training and test sets, respectively. This retrospective study was approved by the Institutional Ethics Review Board; the patients were exempted from signing informed consent.MRI examinations in all patients from institution I and institution II were performed using a 1.5T (Magnetom Aera; Siemens Healthcare, Erlanger, Germany) and 3.0T (Magnetom Trio A Tim; Siemens Healthcare) MR scanner, respectively. The region of interest (ROI) was placed as far as possible in the area with enhancement in lesions to avoid necrosis, hemorrhage, fat, and artifacts. The area of ROI was approximately 1.0~1.5cm2; the same lesion was measured three times with the same ROI, and then average amounts were calculated. Precontrast and postcontrast T1 relaxation times were measured before and 20 min after the contrast medium administration (recorded as T1rt-Pre and T1rt-HBP, respectively), and the reduction rate of T1 relaxation time (rrT1rt) was calculated. In addition, quantitative parameters also included the tumor-to-liver contrast ratio (TLR), tumor enhancement index (TEI), relative tumor enhancement (RTE), relative enhancement ratio (RER), and tumor-to-liver ADC values (recorded as relative ADC, rADC).
Univariable and multivariable logistic regression analyses were performed to investigate the association of clinicoradiological variables and Ki-67 LI. The Kaplan-Meier method was used to evaluate the cumulative recurrence-free survival (RFS). Log rank test was used to evaluate the differences between groups. R software (version 4.1.0) was used for analysis. All differences were considered statistically significant with a p value of <0.05.
Results
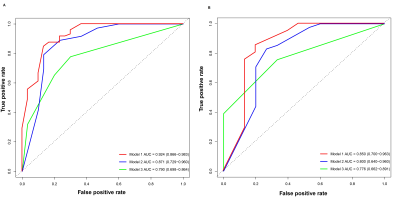
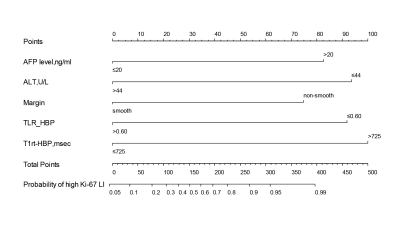
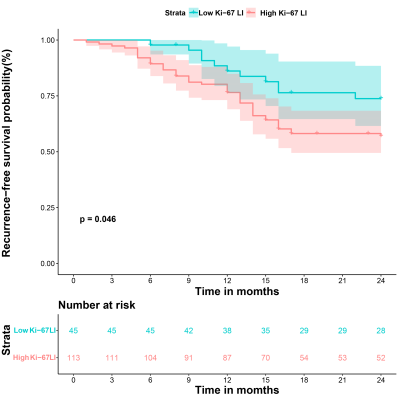
Multivariable analysis showed that alpha-fetoprotein levels > 20ng/ml, alanine aminotransferase > 44 U/L, non-smooth margin, tumor-to-liver signal intensity ratio in the hepatobiliary phase ≤ 0.6, postcontrast T1 relaxation time > 725 msec were significant independent predictors of Ki-67 LI. The combined model constructed based on these significant variables had the best predictive performance with an area under the receiver operator characteristic curve of 0.924, an area under the precision-recall curve of 0.951 and an F1 score of 0.917, and reached 0.850, 0.893, 0.886 in the validation set. RFS rate was significantly lower in the high Ki-67 LI group compared with the low Ki-67 LI group after hepatectomy (24.4% vs. 40.7%, p=0.046).Discussion
Our study showed that elevated preoperative serum AFP level was an independent factor of HCCs with high Ki-67 expression, which is consistent with previous studies [9,10]. In addition, we also found that ALT, NLR and PLR were closely correlated with high Ki-67 LI in HCC. Inflammation has long been recognized as a risk factor for many human cancers [11,12]. Our study found that ALT was an independent factor of high Ki-67 expression in HCC. The possible resolution is that changes in ALT affect the levels of some proinflammatory mediators associated with oncogenic effects, thus accelerating tumor cell proliferation and invasion [13].In our study, the quantitative parameters, including TLR-HBP, TEI-HBP, RER-HBP, TLR-TP, and RER-TP, were based on the SI from either HBP or TP, which may better reveal the Ki-67 LI of HCC given the rationale of gadoxetic acid. Among these quantitative parameters derived from either HBP or TP, HBP-TLR had the highest diagnostic performance in predicting Ki-67 LI of HCCs. The reason why HCCs with higher Ki-67 LI tend to demonstrate lower relative tumoral SI probably is that normal hepatocytes gradually turn into actively proliferated and uncontrolled malignant tumor cells with higher Ki-67 LI during multistep hepatocarcinogenesis, while at the same time, the expression of organic anion transporting polypeptide (OATP) usually decreased, hence resulting in less uptake of gadoxetic acid [14,15].
Furthermore, we also found that gadoxetic acid combined with T1 mapping (T1rt-HBP) had higher predictive performance compared with quantitative parameters derived from SI. T1 relaxation time is an absolute value, which is not affected by scanning sequence parameters, and is proportional to the concentration of gadolinium contrast agent in the tissue [16], while SI is a relative value, the difference of technical factors will affect the value of SI, and does not have a linear relationship with the concentration of contrast agent, so T1 relaxation time is more accurate and reliable than SI.
Conclusion
T1 mapping on gadoxetic acid-enhanced MRI has good predictive efficacy for preoperative prediction of Ki-67 LI, which can promote the individualized risk stratification and further treatment decision of HCC patients.Acknowledgements
We gratefully acknowledge all the members of Department of Radiology, Guangzhou First People’s Hospital, for continuous assistance.References
[1] T. Scholzen, J. Gerdes, The Ki-67 protein: From the known and the unknown, J. Cell. Phys. 182 (2000) 311–322. https://doi.org/10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9.
[2] C. Yang, J. Zhang, M. Ding, K. Xu, L. Li, L. Mao, J. Zheng, Ki67 targeted strategies for cancer therapy, Clin Transl Oncol. 20 (2018) 570–575. https://doi.org/10.1007/s12094-017-1774-3.
[3] K. Murakami, A. Kasajima, N. Kawagishi, N. Ohuchi, H. Sasano, Microvessel density in hepatocellular carcinoma: Prognostic significance and review of the previous published work: MVD in HCC, Hepatol Res. 45 (2015) 1185–1194. https://doi.org/10.1111/hepr.12487.
[4] C.T. Sofocleous, S. Garg, L.M. Petrovic, M. Gonen, E.N. Petre, D.S. Klimstra, S.B. Solomon, K.T. Brown, L.A. Brody, A.M. Covey, R.P. DeMatteo, L. Schwartz, N.E. Kemeny, Ki-67 is a Prognostic Biomarker of Survival after Radiofrequency Ablation of Liver Malignancies, Ann Surg Oncol. 19 (2012) 4262–4269. https://doi.org/10.1245/s10434-012-2461-9.
[5] C. Yang, H. Su, X. Liao, C. Han, T. Yu, G. Zhu, X. Wang, C.A. Winkler, S.J. O’Brien, T. Peng, Marker of proliferation Ki-67 expression is associated with transforming growth factor beta 1 and can predict the prognosis of patients with hepatic B virus-related hepatocellular carcinoma, CMAR. Volume 10 (2018) 679–696. https://doi.org/10.2147/CMAR.S162595.
[6] Y. Luo, F. Ren, Y. Liu, Z. Shi, Z. Tan, H. Xiong, Y. Dang, G. Chen, Clinicopathological and prognostic significance of high Ki-67 labeling index in hepatocellular carcinoma patients: a meta-analysis, (n.d.) 13.
[7] W. Shi, J. Hu, S. Zhu, X. Shen, X. Zhang, C. Yang, H. Zhang, Expression of MTA2 and Ki-67 in hepatocellular carcinoma and their correlation with prognosis, (n.d.) 7.
[8] G. Guzman, V. Alagiozian-Angelova, J.E. Layden-Almer, T.J. Layden, G. Testa, E. Benedetti, A. Kajdacsy-Balla, S.J. Cotler, p53, Ki-67, and serum alpha feto-protein as predictors of hepatocellular carcinoma recurrence in liver transplant patients, Modern Pathology. (2005) 6.
[9] Z. Ye, H. Jiang, J. Chen, X. Liu, Y. Wei, C. Xia, T. Duan, L. Cao, Z. Zhang, B. Song. Texture analysis on gadoxetic acid enhanced-MRI for predicting Ki-67 status in hepatocellular carcinoma: A prospective study, Chinese Journal of Cancer Research. 31 (2019) 806–817. https://doi.org/10.21147/j.issn.1000-9604.2019.05.10.
[10] Y. Fan, Y. Yu, X. Wang, M. Hu, C. Hu, Radiomic analysis of Gd-EOB-DTPA-enhanced MRI predicts Ki-67 expression in hepatocellular carcinoma, BMC Med Imaging. 21 (2021) 100. https://doi.org/10.1186/s12880-021-00633-0.
[11] C.I. Diakos, K.A. Charles, D.C. McMillan, S.J. Clarke, Cancer-related inflammation and treatment effectiveness, The Lancet Oncology. 15 (2014) e493–e503. https://doi.org/10.1016/S1470-2045(14)70263-3.
[12] R. Khandia, A. Munjal, Interplay between inflammation and cancer, in: Advances in Protein Chemistry and Structural Biology, Elsevier, 2020: pp. 199–245. https://doi.org/10.1016/bs.apcsb.2019.09.004.
[13] X. Fu, W. Zhang, S. Li, N. Ling, Y. Yang, Z. Dazhi, Identification of alanine aminotransferase 1 interaction network via iTRAQ-based proteomics in alternating migration, invasion, proliferation and apoptosis of HepG2 cells, Aging. 14 (2022) 7137–7155. https://doi.org/10.18632/aging.204286.
[14] J. Chen, C. Chen, C. Xia, Z. Huang, P. Zuo, A. Stemmer, B. Song, Quantitative free-breathing dynamic contrast-enhanced MRI in hepatocellular carcinoma using gadoxetic acid: correlations with Ki67 proliferation status, histological grades, and microvascular density, Abdom Radiol. 43 (2018) 1393–1403. https://doi.org/10.1007/s00261-017-1320-3.
[15] Z. Ye, L. Cao, Y. Wei, J. Chen, Z. Zhang, S. Yao, T. Duan, B. Song, Preoperative prediction of hepatocellular carcinoma with highly aggressive characteristics using quantitative parameters derived from hepatobiliary phase MR images, Ann Transl Med. 8 (2020) 85–85. https://doi.org/10.21037/atm.2020.01.04.
[16] C. Rao, X. Wang, M. Li, G. Zhou, H. Gu, Value of T1 mapping on gadoxetic acid-enhanced MRI for microvascular invasion of hepatocellular carcinoma: a retrospective study, BMC Med Imaging. 20 (2020) 43. https://doi.org/10.1186/s12880-020-00433-y.
Figures