0718
Prediction of the recurrence and invasion of hepatocellular carcinoma after TACE using intravoxel incoherent motion1Department of Radiology, Yantaishan Hospital, Yantai , Shandong Province, China, 2GE Healthcare, MR Research China, Beijing, China, 3Shandong Province Qianfoshan Hospital, Jinan, Shandong Province, China
Synopsis
Keywords: Liver, Diffusion/other diffusion imaging techniques, IVIM
The accurate evaluation of tumor response after Transarterial Chemoembolization (TACE) treatment is important for tumor prognosis and subsequent treatment. In this study, we comprehensively investigated the feasibility of IVIM parameters in the TACE treatment area, peritumoral area, and hepatic parenchymal to predict tumor recurrence in hepatocellular carcinoma patients after TACE treatment. ADC and D of TACE-treated area, and D of the peritumoral area can predict the intralesional and peritumoral recurrence after TACE treatment with high accuracy, indicating that IVIM might be a useful tool in predicting the therapeutic response and the peritumoral invasion.Introduction
Transarterial Chemoembolization (TACE) is widely used for the treatment of hepatocellular carcinoma (HCC) patients who were not suitable for radical treatments. (1) However, there is still a high incidence of local tumor recurrence following treatment with TACE. (2) The accurate detection of intralesional and peritumoral recurrence plays a crucial role in the follow-up process. (3) Previous studies have reported that IVIM might be a useful tool for predicting the response of HCC to TACE. (4,5) However, these studies mainly focused on the prediction effect of IVIM parameters in the TACE-treated area, and the results were not consistent. Limited studies have comprehensively compared the prediction power of IVIM parameters in the TACE-treated area, peritumoral area, and the parenchyma of the liver in detecting the intralesional and peritumoral recurrence after TACE treatment. This study aimed to systematically evaluate the clinical feasibility of IVIM-derived metrics in predicting the therapeutic response of HCC to TACE, in order to facilitate the formulation of more aggressive therapeutic strategies.Methods
This prospective study involved 47 HCC patients who were previously treated with TACE between January 2018 to December 2021. The MR imaging was performed on a 3.0 T system (Discovery MR750, GE Healthcare, MI, United States) with an eight-channel abdomen coil. Before the MR examination, each patient was instructed to fast for six to eight hours. IVIM-DWI was performed in an axial plane using a respiratory-gated spin-echo echo-planar imaging sequence (SE-EPI). 10 different b values between 0 and 1000 s/mm2 (25, 50, 75, 100, 200, 400, 600, 700, 800, and 1000) were used. The other parameters of IVIM-DWI were as follows: repetition time ranged from 4000 to 20000, echo time 52.9ms, bandwidth 250kHz/pixel, acquisition matrix 128×130, field of view 360mm×288mm, slice thickness 8mm, and slice gap 2mm. In the follow-up, according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) (6), the patients were divided into two groups – progressive and non-progressive groups. ADC and IVIM-derived parameters (D, D*, f) were calculated on workstation 4.6 (GE Healthcare, USA). The regions of interests (ROIs) were manually drawn on the original images (b=0) of the IVIM, including ROI of the tumor solid area, ROI of the peritumoral area (distance<2 cm to the tumor boundary), and ROI of the liver parenchyma (distance>5 cm from the tumor boundary). Then the four parameter values in the three ROIs can be obtained. In addition, considering the inter-subject variation and individual differences, the parameters of the peritumoral zone were normalized by those of the non-tumor liver parenchyma, e.g., ADCstd= ADCpt/ADClp, ADCpt and ADClp represented the mean ADC values in the peritumoral and liver parenchyma. The statistical analyses were conducted using SPSS software (IBM Corp., Armonk, NY, USA) and MedCalc software (v. 19.2.0 for Windows, Mariakerke, Belgium). Using an independent sample t-test, IVIM metrics were compared in the non-progressing groups and the progression groups. Receiver operating characteristic (ROC) curves were used to evaluate the ability of ADC, D, D*, and f to distinguish between the progressive and non-progressive groups. For all statistical tests, P<0.05 was considered to be statistically significant.Results
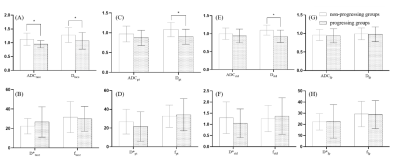
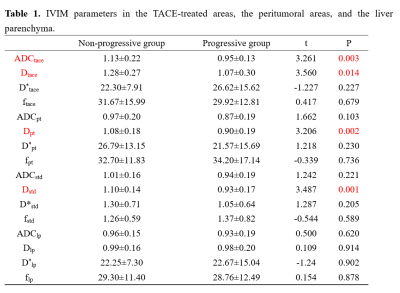
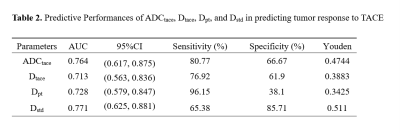
Table 1 and Fig. 1 show the mean values of ADC, D, D*, and f in different ROIs and the corresponding t-test results between the progressive and non-progressive groups. Compared to the progressive group, the non-progressive group showed greater values of ADCtace and Dtace in the TACE-treated areas (P<0.05). Similarly, there were significant differences between Dpt in the peritumoral area and normalized parameter Dstd in the progressive and non-progressive groups. As shown in Table 2, the AUC values of ADCtace, Dtace, Dpt, and Dstd were 0.76, 0.71, 0.73, and 0.77, respectively.Discussion
It was found that ADCtace, Dtace, Dpt, and Dstd showed statistically significant differences between the non-progressing groups and progressing groups (P<0.05). In addition, Dstd in the peritumoral area showed a high predictive power (AUC = 0.77) in predicting tumor response after TACE treatment, indicating that Dstd may be useful for predicting the cellular invasion of the peritumoral liver zone. Ghadery et al. (5) and Fei Jia et al. (4) reported that the D value of the tumor region in the non-progressive group was higher than that in the progressive group, which was consistent with this study. When the tumor recurred, the cell density and the nuclear-to-cytoplasmic ratio would increase, (7,8)resulting in limited diffusion of water, which leads to the decrease of ADC and D values. There were no significant differences in the Dlp*, Dlp, and flp of the hepatic parenchyma between the two groups, suggesting that TACE seemed not to have a significant effect on the normal liver parenchyma.Conclusion
This study showed that ADCtace and Dtace in the TACE-treated area, Dpt and normalized parameter Dstd in the peritumoral tissues can predict the therapeutic response of TACE with relatively high accuracy, indicating that IVIM-derived parameters may be helpful to evaluate HCC response to TACE, and sensitive for monitoring perilesional tumor recurrence.Acknowledgements
No acknowledgement found.References
1. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 2016;64(1):106-116.
2. Rou WS, Lee BS, Moon HS, Lee ES, Kim SH, Lee HYJWJoGW. Risk factors and therapeutic results of early local recurrence after transcatheter arterial chemoembolization. 2014;20(22):6995.
3. Takayasu K, Arii S, Ikai I, Kudo M, Matsuyama Y, Kojiro M, Makuuchi M, Liver Cancer Study Group of J. Overall survival after transarterial lipiodol infusion chemotherapy with or without embolization for unresectable hepatocellular carcinoma: propensity score analysis. AJR Am J Roentgenol 2010;194(3):830-837.
4. Jia F, Wu B, Yan R, Li L, Wang K, Han DJJoMRI. Prediction Model for Intermediate‐Stage Hepatocellular Carcinoma Response to Transarterial Chemoembolization. 2020;52(6):1657-1667.
5. Ghadery AH, Yazdi NA, Bagheri H, Kazerooni AF, Salahshour F, Javidi SS, Saeedi S, Rad HS, Shekarchi BJEJoR, Medicine N. Prediction of hepatocellular carcinoma response to transarterial chemoembolization with intravoxel incoherent motion diffusion-weighted imaging. 2022;53(1):1-8.
6. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30(1):52-60.
7. Jia F, Wu B, Yan R, Li L, Wang K, Han D. Prediction Model for Intermediate-Stage Hepatocellular Carcinoma Response to Transarterial Chemoembolization. J Magn Reson Imaging 2020;52(6):1657-1667.
8. Zhang Y, Kuang S, Shan Q, Rong D, Zhang Z, Yang H, Wu J, Chen J, He B, Deng Y, Roberts N, Shen J, Venkatesh SK, Wang J. Can IVIM help predict HCC recurrence after hepatectomy? Eur Radiol 2019;29(11):5791-5803.
Figures