0706
Deep learning-based segmentations challenge established link between stroke volume and functional outcome after thrombectomy
Ingrid Digernes1, Martin Soria Røvang1, Terje Nome2, Cecilie Nome3, Thor Håkon Skattør2, Brian Anthony Enriquez3, Bradley J MacIntosh1, Anne Hege Aamodt3, and Atle Bjørnerud1
1Computational Radiology and Artificial Intelligence, Oslo University Hospital, Oslo, Norway, 2Radiology and Nuclear Medicine, Oslo University Hospital, Oslo, Norway, 3Department of Neurology, Oslo University Hospital, Oslo, Norway
1Computational Radiology and Artificial Intelligence, Oslo University Hospital, Oslo, Norway, 2Radiology and Nuclear Medicine, Oslo University Hospital, Oslo, Norway, 3Department of Neurology, Oslo University Hospital, Oslo, Norway
Synopsis
Keywords: Stroke, Machine Learning/Artificial Intelligence, segmentation, functional outcome, thrombectomy
Using a standard 3D nnUNET model that was pretrained to segment WMH on FLAIR, we obtained good stroke lesion segmentation accuracy (median Dice = 0.80) when the model was re-trained to segment the stroke lesion on DWI in a smaller stroke sample (N = 82). Applying the segmentation model to DWIs from 307 thrombectomy patients, we found no association between pre-treatment lesion volume and functional outcome at 90 days after thrombectomy. Our results indicate that the established assumption of lesion size being a strong predictor of functional outcome should be investigated further for patients receiving mechanical thrombectomy.Introduction
In acute ischemic stroke (AIS), a blood clot hinders the blood supply to a brain region, causing irreversible damage to the brain tissue if not acutely treated. The size of the core infarct is known to be a strong predictor of functional outcome after AIS.1 A large infarct core (> 70 mL) at initial presentation has been viewed as grounds for exclusion in clinical trials that evaluate the efficacy of endovascular thrombectomy.2,3 In this context, there is great interest to understand the relationship between stroke volume and outcomes.Diffusion weighted imaging (DWI) provides superior imaging contrast to assess the infarct core.4 Manual segmentation of lesion volume on DWI is needed and is time consuming. In both clinical and research settings, lesion size assessment is usually performed with faster, but less accurate methods, such as Alberta Stroke Program Early CT (ASPECT) score.
Deep learning-based methods potentially offers fast and accurate stroke segmentation from DWI, but generally require large training sets of expert annotated data, which is challenging to obtain.5 Here, we examine whether a deep learning-based acute stroke segmentation will perform well when considering a relatively small training dataset and after initializing the model weights by training on a large non-acute stroke cohort. We further investigate the association between estimated stroke volume and functional outcome after thrombectomy in a larger cohort.
Methods
Deep learning model:The deep learning model used a 3D nn-UNET network that was pre-trained on in-house Fluid Attenuation Inversion Recovery (FLAIR) images and ground truth white matter hyperintensity (WMH) lesion segmentations.6 The stroke sample consisted of DWI from a subset of stroke patients in the Oslo Acute Revascularization Stroke Study (OSCAR) who underwent thrombectomy at the Oslo University Hospital. Hence, the 3D nn-UNET was initially trained on FLAIR-based WMH (N=582) and then DWI-based stroke. The DWI data were acquired before and 24-hours after treatment (N=51 patients, N=102 DWIs). The DWI data were manually annotated by two expert neuroradiologist and one junior radiology trainee. A test set of N = 20 images was kept outside the training procedure. The remaining data (N = 82) was split into a training (N = 65) and a validation set (N = 17). The model’s performance was evaluated by the median ± interquartile range of the Dice score across subjects in the validation and test datasets.
Clinical analysis:
The trained nn-UNET was deployed to segment stroke lesion on pre-treatment DWI from a new subset of thrombectomy patients (N = 307) from the OSCAR study. Functional outcome was defined as good if the patient had modified Rankin Score (mRS) < = 2 at 90 days after treatment, and poor if mRS > 2.
Results
The pre-trained 3D nn-UNET was trained for 23 additional epochs and obtained a Dice score of 0.913 ± 0.084 in the validation set, and 0.800 ± 0.272 in the test set. A histogram showing Dice scores for N=102 stroke segmentation cases is shown in Figure 1.Smaller stroke lesions tended to yield lower dice scores (< 0.6), as seen in Figure 2. Considering lesions with volume > 20 mL, the median Dice score of the test set was 0.873 ± 0.099. Test set segmentation examples are shown in Figure 3, with a small lesion and low Dice in (a) and large lesion and high Dice in (b).
In the clinical analysis, we found a mean lesion volume at admission of 15.1 mL for patients with good functional outcome and 18.4 mL for poor functional outcome, but the difference was not significant (two-sample T-test, P = 0.104; Figure 4)
Moreover, no significant higher risk for poor functional outcome was found when stratifying patients based on lesion volume (volume ≤ 70 mL vs. volume > 70 mL) (chi-square test P = 0.211).
Discussion and conclusion
Using a standard 3D nnUNET model pretrained to segment WMH on FLAIR, we obtained good stroke lesion segmentation accuracy when the model was re-trained to segment stroke lesions on DWI in a smaller stroke sample (N = 82). We achieved a model with median Dice score of 0.91 and 0.80 in the validation and test sets, respectively. Dice scores increased with increasing lesion volume, as expected, and more training data with small lesion volumes could remedy this bias.To our knowledge, the current state-of-the-art stroke lesion segmentation model has a test set Dice score = 0.858 (using both ADC and DWI as input and with a dataset of approximately N = 7000).7 Whereas other DWI stroke segmentation models recently published have similar or lower Dice scores than our current model (test set Dice from 0.67 to 0.81).8-10
Applying the segmentation model to DWIs from 307 thrombectomy patients, we found no association between pre-treatment lesion volume and functional outcome after thrombectomy. This result is at odds with the established view, and some explanations are warranted, for instance, selection bias and the proportion of patient receiving thrombectomy may be factors. Some recent studies examining functional outcome in thrombectomy patients are in accordance with our findings.11,12
To conclude, our results indicate that the established assumption of lesion size being a strong predictor of functional outcome should be investigated further for thrombectomy patients.
Acknowledgements
No acknowledgement found.References
- Yoo AJ, Barak ER, Copen WA, et al. Combining acute diffusion-weighted imaging and mean transmit time lesion volumes with National Institutes of Health Stroke Scale Score improves the prediction of acute stroke outcome. Stroke. 2010;41(8):1728-1735. doi:10.1161/STROKEAHA.110.582874
- Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association [published correction appears in Stroke. 2018 Mar;49(3):e138] [published correction appears in Stroke. 2018 Apr 18;:]. Stroke. 2018;49(3):e46-e110. doi:10.1161/STR.0000000000000158
- Román LS, Menon BK, Blasco J, et al. Imaging features and safety and efficacy of endovascular stroke treatment: a meta-analysis of individual patient-level data [published correction appears in Lancet Neurol. 2018 Dec;17(12):e2-e3]. Lancet Neurol. 2018;17(10):895-904. doi:10.1016/S1474-4422(18)30242-4
- El-Koussy M, Schroth G, Brekenfeld C, Arnold M. Imaging of acute ischemic stroke. Eur Neurol. 2014;72(5-6):309-316. doi:10.1159/000362719
- Prevedello LM, Halabi SS, Shih G, et al. Challenges Related to Artificial Intelligence Research in Medical Imaging and the Importance of Image Analysis Competitions. Radiol Artif Intell. 2019;1(1):e180031. Published 2019 Jan 30. doi:10.1148/ryai.2019180031
- Røvang MS, Selnes P, MacIntosh BJ, et al. Segmenting white matter hyperintensities on isotropic three-dimensional Fluid Attenuated Inversion Recovery magnetic resonance images: A comparison of Deep learning tools on a Norwegian national imaging database. 2022. arXiv preprint arXiv:2207.08467.
- Alis D, Yergin M, Alis C, et al. Inter-vendor performance of deep learning in segmenting acute ischemic lesions on diffusion-weighted imaging: a multicenter study. Sci Rep. 2021;11(1):12434. Published 2021 Jun 14. doi:10.1038/s41598-021-91467-x
- Chen L, Bentley P, Rueckert D. Fully automatic acute ischemic lesion segmentation in DWI using convolutional neural networks. Neuroimage Clin. 2017;15:633-643. Published 2017 Jun 13. doi:10.1016/j.nicl.2017.06.016
- Winzeck S, Mocking SJT, Bezerra R, et al. Ensemble of Convolutional Neural Networks Improves Automated Segmentation of Acute Ischemic Lesions Using Multiparametric Diffusion-Weighted MRI. AJNR Am J Neuroradiol. 2019;40(6):938-945. doi:10.3174/ajnr.A6077
- Liu CF, Hsu J, Xu X, et al. Deep learning-based detection and segmentation of diffusion abnormalities in acute ischemic stroke. Commun Med (Lond). 2021;1:61. Published 2021 Dec 16. doi:10.1038/s43856-021-00062-8
- Kerleroux B, Janot K, Dargazanli C, et al. Perfusion Imaging to Select Patients with Large Ischemic Core for Mechanical Thrombectomy. J Stroke. 2020;22(2):225-233. doi:10.5853/jos.2019.02908
- Wang J, Qiu J, Wang Y. Neurological Functional Independence After Endovascular Thrombectomy and Different Imaging Modalities for Large Infarct Core Assessment : A Systematic Review and Meta-analysis [published online ahead of print, 2022 Aug 3]. Clin Neuroradiol. 2022;10.1007/s00062-022-01202-w. doi:10.1007/s00062-022-01202-w
Figures
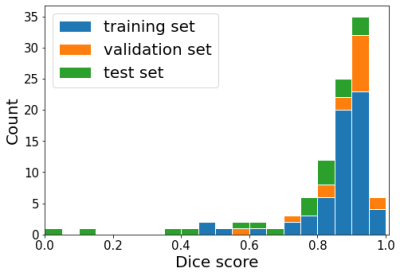
Figure 1: Histogram of the Dice scores across the training set (blue), validation set (orange) and test set (green).
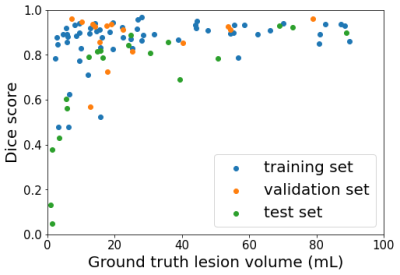
Figure 2: Scatter plot of Dice score vs ground truth lesion volume where each dot represents one segmentation from the training set (blue), validation set (orange) and test set (green).
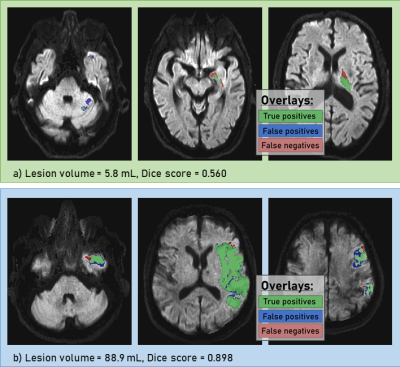
Figure 3: Segmentation examples from the test set. Top row (a) displays results from a patients with small lesion (volume = 5.8 mL) and bottom row (b) displays results from a large lesion (volume = 88.9 mL). Green overlay represents voxels correctly identified as ischemic core, blue overlay represents false positive voxels and red represents false positive voxels.
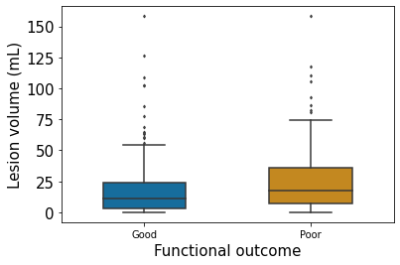
Figure 4: Boxplot of lesion volume vs. functional outcome.
DOI: https://doi.org/10.58530/2023/0706