0699
Large vessel occlusion detection on TOF images using deep learning1OLEA MEDICAL, La Ciotat, France
Synopsis
Keywords: Stroke, Machine Learning/Artificial Intelligence, Large vessel occlusion, TOF, MR angiography
Large vessel occlusion (LVO) in stroke patients is mostly detected using deep-learning-based automated methods on CT angiography but there has been no report on such methods using time-of-flight magnetic resonance angiography (TOF-MRA). Our study includes 460 stroke patients with 230 LVO-positive cases. The first step was vessel segmentation, and the output mask was used for the LVO detection. Both steps were deep-learning based using TOF-MRA. Our model successfully detected 95% LVO-positive and 92% LVO-negative patients. The high detection rate and short processing time (< 60 seconds) suggested that our model is highly adequate in a clinical emergency context.Introduction
Large vessel occlusion (LVO) of the anterior circulation causes three-fifth of poststroke dependence and death1. Recent studies have shown that, when treated within 24 hours after the early stroke symptoms, the clinical outcome of LVO patients is significantly better after a successful endovascular clot retrieval (ECR) than those treated medically1–6, hence the importance of robuste LVO detection methods. CT angiography (CTA) is currently the most widely used imaging modality for deep-learning based LVO detection methods in stroke patients7–20. To the best of our knowledge, however, there has been no report of deep learning LVO detection algorithm based on time-of-flight magnetic resonance angiography (TOF-MRA). The aim of this study is therefore to propose a new two-steps deep-learning-based algorithm allowing to automatically segment cerebral vasculature and subsequently detect LVO presence in TOF-MRA data.Methods
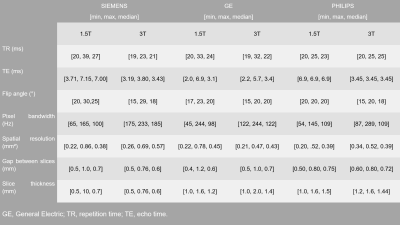
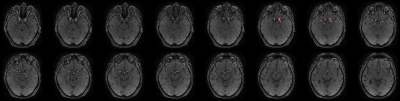
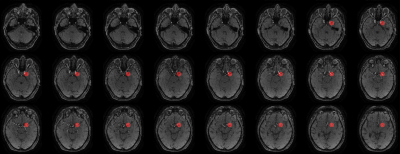
Anonymized data from a total of 460 patients were included in this retrospective multicentric study (50% female). All patients underwent a standard stroke MRI examination after signing a consent form. TOF sequence parameters are listed in Figure 1. TOF images were analyzed by 4 experts, focusing on anterior circulation occlusions (M1 and M2), 230 among them were positive. All experiments were performed on 64-bit Windows 10 operating system with a 6-core Intel(R) Core (TM) i7-8700k CPU @ 3.70GHz, 32 GB RAM and the NVIDIA GeForce GTX 1080 graphic unit. The home-built framework was based on Python (version 3.7) and the deep learning part was adapted from Monai (version 0.8.0). Data labelling and manual correction of segmentations were performed using ITK-Snap software (version 3.8.0). The two-steps post-processing pipeline relied on automated vessel segmentation and LVO detection. For vessel segmentation, a subset of 300 cases was used. The ground truth was generated semi-manually using an in-house python implementation of the Hessian-based Frangi vesselness filter21 on the TOF volume of 45 cases, then manually corrected using ITK-Snap. The ground-truth for the remaining cases was generated using the CNN model pre-trained with the first segmented dataset then manually corrected. For the training, we used the Monai-implemented BasicUnet model with the Dice Cross-Entropy loss, and the Adam optimizer. For LVO detection, a total dataset of 230 LVO-positive cases and 230 LVO-negative cases were used. Database was split into 70%, 20%, 10% for training, validation, and test, respectively. Vessels around the occlusion site, in LVO-positive cases, were segmented by experts on TOF images, on 10 slices before and after the occlusion location (Figure 2). Circular masks of 20mm diameter were subsequently placed over 20 slices around the centroid of the experts’ masks (Figure 3) and used as ground-truth. The vessel segmentation from the previous step was used as an additional mask for the training which was performed using the BasicUnet model with features (64, 128, 256, 512, 1024), a 0.6 dropout rate to overcome overfitting and Focal Loss. The algorithm’s outputs are a binary mask of a circle locating the predicted occlusion site and the 3D coordinates of its centroid.Results
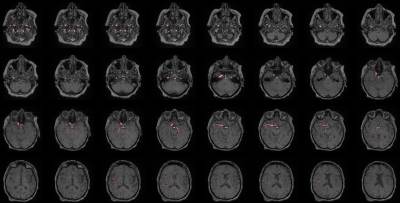
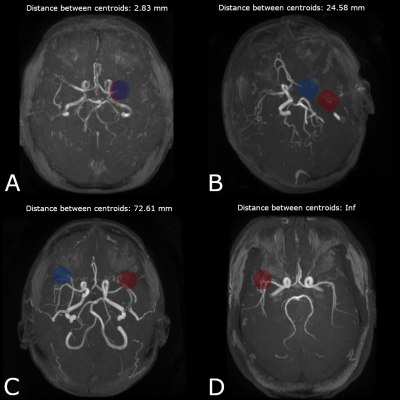
The vessel segmentation model showed satisfactory performance, both qualitatively and quantitatively, with median [min- max] values of 0.911 [0.582 - 0.973] and 0.906 [0.705 - 0.969] for Dice and Tversky, respectively. Visual assessment of the segmentation showed that in all patients, large vessels were correctly segmented, whereas some small vessels were missed by the algorithm (see Figure 4). In some patients, false positive voxels were observed around the skin zone, leading to lower metrics. For LVO detection, algorithm performance assessment was based on the distance between the centroids of the ground truth mask and the predicted one. The model successfully detected the occlusion site (distance between the centroids < 20mm) in 95% of the cases (Figure 5, A). For the remaining 5%, occlusion location was detected on the wrong hemisphere or the distance between centroids was > 20 mm (Figure 5, B&C). Among the LVO-positive patients from the test dataset, 3.6% were false negative predictions (Figure 5, D) and successfully classified 92% of the tested LVO-negative cases. The model provided 95% sensitivity, 92% specificity and 93% accuracy.Discussion
CTA-based studies evaluating diagnostic performances of both human readers and deep-learning-based algorithms generally report good LVO detection rates for occlusions in the ICA and M1 segments, but a slightly lower efficiency in M2 segments. For human readers, sensitivities ranging from 89% to 97% have been reported for LVO detection in ICA and M1 segments, whereas, reported deep-learning models showed sensitivities of 92% and specificities of 81%. Our model, based on TOF-MRA, provided high performance in both LVO-positive and LVO-negative detection, with sensitivity, specificity, and accuracy of 95%, 92% and 93% respectively, thus outperforming CTA-based models for anterior circulation occlusions site localization.Conclusion
The objective of this study was to propose and evaluate the performance of a new automated deep-learning-based algorithm to detect large vessel occlusion using TOF-MRA data. The results demonstrated an overall high detection rate, particularly for M1 and M2 segments. The short processing time (< 60 seconds) suggested that this algorithm is highly adequate in a clinical emergency context.Acknowledgements
No acknowledgement found.References
1. Malhotra, K., Gornbein, J. & Saver, J. L. Ischemic Strokes Due to Large-Vessel Occlusions Contribute Disproportionately to Stroke-Related Dependence and Death: A Review. Front. Neurol. 8, (2017).
2. Campbell, B. C. V. et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. N. Engl. J. Med. 372, 1009–1018 (2015).
3. Goyal, M. et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N. Engl. J. Med. 372, 1019–1030 (2015).
4. Berkhemer, O. A. et al. A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. N. Engl. J. Med. 372, 11–20 (2015).
5. Nogueira, R. G. et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 378, 11–21 (2018).
6. Albers, G. W. et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 378, 708–718 (2018).
7. Lee, H. et al. An explainable deep-learning algorithm for the detection of acute intracranial haemorrhage from small datasets. Nat. Biomed. Eng. 3, 173–182 (2019).
8. Ginat, D. T. Analysis of head CT scans flagged by deep learning software for acute intracranial hemorrhage. Neuroradiology 62, 335–340 (2020).
9. Frölich, A. M. J. et al. Angiographic reconstructions from whole-brain perfusion CT for the detection of large vessel occlusion in acute stroke. Stroke 43, 97–102 (2012).
10. Stib, M. T. et al. Detecting large vessel occlusion at multiphase CT angiography by using a deep convolutional neural network. Radiology 297, 640–649 (2020). 11. Amukotuwa, S. A., Straka, M., Dehkharghani, S. & Bammer, R. Fast automatic detection of large vessel occlusions on CT angiography. Stroke 50, 3431–3438 (2019).
12. Amukotuwa, S. A. et al. Automated detection of intracranial large vessel occlusions on computed tomography angiography a single center experience. Stroke 50, 2790–2798 (2019).
13. Reidler, P. et al. Performance of Automated Attenuation Measurements at Identifying Large Vessel Occlusion Stroke on CT Angiography. Clin. Neuroradiol. 31, 763–772 (2021).
14. Rava, R. A. et al. Validation of an artificial intelligence-driven large vessel occlusion detection algorithm for acute ischemic stroke patients. Neuroradiol. J. 34, 408–417 (2021). 15. Dehkharghani, S. et al. High-performance automated anterior circulation CT angiographic clot detection in acute stroke: A multireader comparison. Radiology 298, 665–670 (2021).
16. Luijten, S. P. R. et al. Diagnostic performance of an algorithm for automated large vessel occlusion detection on CT angiography. J. Neurointerv. Surg. neurintsurg-2021-017842 (2021) doi:10.1136/neurintsurg-2021-017842.
17. Seker, F. et al. Diagnostic accuracy of automated occlusion detection in CT angiography using e-CTA. Int. J. Stroke 17, 77–82 (2022).
18. Murray, N. M., Unberath, M., Hager, G. D. & Hui, F. K. Artificial intelligence to diagnose ischemic stroke and identify large vessel occlusions: A systematic review. J. Neurointerv. Surg. 12, 156–164 (2020).
19. McLouth, J. et al. Validation of a Deep Learning Tool in the Detection of Intracranial Hemorrhage and Large Vessel Occlusion. Front. Neurol. 12, 1–10 (2021).
20. Chang, P. D. et al. Hybrid 3D/2D convolutional neural network for hemorrhage evaluation on head CT. Am. J. Neuroradiol. 39, 1609–1616 (2018).
21. Frangi, A. F., Niessen, W. J., Vincken, K. L. & Viergever, M. A. Multiscale vessel enhancement filtering. Lect. Notes Comput. Sci. (including Subser. Lect. Notes Artif. Intell. Lect. Notes Bioinformatics) 1496, 130–137 (1998).
Figures