0695
Highly accelerated FLEXA 3DTOF MR Angiography with iterative deep learning reconstruction1GE Healthcare, Tokyo, Japan, 2GE Research, Herzliya, Israel, 3GE Research, Niskayuna, NY, United States, 4GE Healthcare, Waukesha, WI, United States, 5GE Healthcare, Menlo Park, CA, United States, 6Keio University School of Medicine, Tokyo, Japan
Synopsis
Keywords: Vessels, Machine Learning/Artificial Intelligence
Rapid non-contrast MRA of supra-aortic arteries is necessary to select proper patient for endovascular therapy (EVT) as EVT has become the predominant therapy of acute ischemic stroke. However, conventional 3DTOF has long scan time of 6-7 minutes to cover the entire carotid artery. A highly accelerated MRA with iterative deep learning reconstruction was developed to provide the wide scan coverage and less motion sensitivity within 1 minute. We evaluated the technique with prospectively and retrospectively undersampled data demonstrating high-quality vessel visualization and improved acquisition efficiency at 8x acceleration.Introduction
Vessel occlusion arising from supra-aortic arteries is a common source of cerebral stroke and infarction1. We have been developing an accelerated non-contrast 3DTOF MOSTA MRA of two-point Dixon based acquisition called FLEXA2,3 to provide the wide scan coverage and less motion sensitivity in a 2-minute scan time. It is expected to be alternative to conventional 3D TOF that has been clinically used to visualize vessels of carotid artery with the long scan time of 6-7 minutes limiting the imaging coverage of carotid bifurcation. However, to select for patients eligible for endovascular treatment in acute stroke, scan protocol should be practical and as fast as possible4. In this work, we explore the feasibility and performance of a deep learning-based reconstruction for further scan acceleration.Methods
For deep learning-based image reconstruction (DLR), we used DCI-Net (Densely Connected Iterative Network)5with 28 iterations, 9 convolutional layers per iteration, 96 2D convolution kernels (applied to phase-slice encoding planes) per layer, and up to 20 skip connections per iteration. The DCI-Net was trained using 149 3D T1-weighted brain data sets. For the loss function, we used a contrast-weighted structural similarity (SSIM)6 extended to complex-valued images where the weights for the luminance, contrast, and structure comparison functions were 0.3, 1, and 0.3, respectively.To evaluate the performance of the DLR in FLEXA, k-space data were retrospectively undersampled from fully-sampled data. Averaged SSIM and mean squared error (MSE) were calculated for all slices to compare undersampled images to full-sampled images as the reference standard. The acceleration factors of 2,4,6,8,10 and 12 were used with variable density poisson disk patterns.
To prospectively evaluate image quality, FLEXA scans were performed at the DLR accelerations of 4.4, 6.0 and 8.0 and compared with the combined ARC7 parallel imaging and compressed sensing8 scan at 4.4x acceleration. FLEXA scan was also performed at 8.4x acceleration to compare to ARC parallel imaging scan alone at the same acceleration. In addition, the DLR reconstruction was used with the sampling pattern of the parallel imaging. Healthy volunteer scans were performed under the IRB approval. A 3.0 T System (Signa Pioneer, GE Healthcare, Waukesha, WI, U.S.A.) with Head and Neck 21 channel coils (GE Healthcare) was used. FLEXA uses ramped RF pulse. For the FLEXA reconstruction, the Dixon reconstruction uses FLEX9 to separate water (W) and fat (F) images from in-phase (IP) and out-of-phase (OP) images. A composite image is calculated by to compensate for Water-Fat swap that is the incorrect assignment of water and fat components. FLEXA scans of the neck MRA were performed with the following protocol: flip angle: 10 °, TR/TE: 7.8/1.5 ms, receiver bandwitdth: 166.7 kHz, FOV: 30 cm, slice thickness: 2cm, number of slabs: 15-16, spatial resolution: 1.3x1.0x2.0 cm.
Results
Figure 2 shows MSE and averaged SSIM index with different acceleration factors for the retrospectively undersampled data. As the acceleration factors was increased, the MSE and SSIM index gradually deteriorated, maintaining a high value of 0.97 at DL 10x acceleration for the SSIM index.Figure 3 shows representative images of the prospective evaluation compared to the combined parallel imaging and compressed sensing images. The accelerated DLR scans had comparable vessel depictions from aortic arch to the whole neck to the combined parallel imaging and compressed sensing in MIP images.
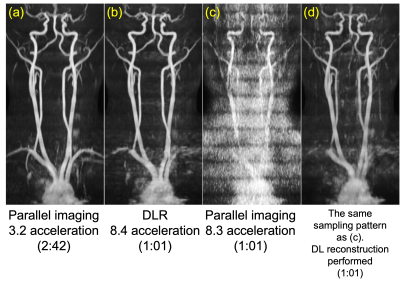
Figure 4 shows representative images compared to the ARC parallel imaging alone of 3.2x acceleration. The DLR 8.4x acceleration gave comparable vessel delineation of the whole neck to ARC 3.2x acceleration. Vessels on neck-chest region was not visualized well on the DLR image because the frequency encoding direction was AP in a wrong scan prescription, causing wrap-around with shoulders. Using the DL reconstruction was able to recovery a significant portion of missing data with regularly spaced k-space points at R=9 (acquire one, skip eight to create sampling reduction), while parallel imaging reconstruction failed.
Discussions and conclusion
The DLR FLEXA achieved high-quality 1-minute whole neck MRA, offering a promising approach to confirm the presence of a large vessel occlusion before performing endovascular therapy for acute stroke. The DCI-Net helps reach the full potential of FLEXA to improve the acquisition efficiency even when FLEXA uses thin slab acquisition technique that limits the spatially varying coil sensitivity along slice encoding direction and allows undersampled data along only the phase encoding direction in ky-kz plane for parallel imaging. Additionally, the highly accelerated FLEXA scan would contribute to reducing the sensitivity to motion artifact such as swallowing and breathing, leading to robust image quality. Clinical studies are warranted to validate its technique.Acknowledgements
No acknowledgement found.References
1. Phan T, Huston J III, Bernstein MA, Riederer SJ, Brown RD Jr. Contrast-enhanced magnetic resonance angiography of the cervi- cal vessels: experience with 422 patients. Stroke 2001;32: 2282–2286.
2. Amemiya S, Takei N, Ueyama T, Fujii K, Takao H, Yasaka K, Watanabe Y, Kamiya K, Abe O. Accelerated Two-Point Dixon MR Angiography Improves Diagnostic Performance for Cervical Artery Diseases. J Magn Reson Imaging. 2022 Sep;56(3):929-941.
3. Takei N, Amemiya S et al. FLEXA 3DTOF: Fast 3D TOF MR Angiography using Thin-slab Two-Point Dixon Acquisition. In Proceedings of the Joint annual meeting of the ISMRM-ESBRMB 4130 (2022).
4. van der Zijden T, Mondelaers A, Yperzeele L, Voormolen M, Parizel PM. Current concepts in imaging and endovascular treatment of acute ischemic stroke: implications for the clinician. Insights Imaging. 2019 Jun 13;10(1):64.
5. Malkiel, I., Ahn, S., Slavens, Z., Taviani, V. & Hardy, C. J. Densely Connected Iterative Network for Sparse MRI Reconstruction. in Proceedings of the 27th Joint annual meeting of the ISMRM-ESMRMB 3363 (2018).
6. Ahn S, Menini A, McKinnon G, et al. Contrast-weighted SSIM loss function for deep learning-based undersampled MRI reconstruction. in Proceedings of the 28th annual meeting of the ISMRM 1295 (2020).
7. Brau AC, Beatty PJ, Skare S, Bammer R. Comparison of reconstruction accuracy and efficiency among autocalibrating data‐driven parallel imaging methods. Magn Reson Med 2008; 59: 382– 395.
8. King et al. A New Combination of Compressed Sensing and Data Driven Parallel Imaging. In Proceedings of the 18th annual meeting of the ISMRM 4881 (2010).
9. Ma J. Breath-hold water and fat imaging using a dual-echo two- point Dixon technique with an efficient and robust phase-correction algorithm. Magn Reson Med 2004;52:415–419.
Figures
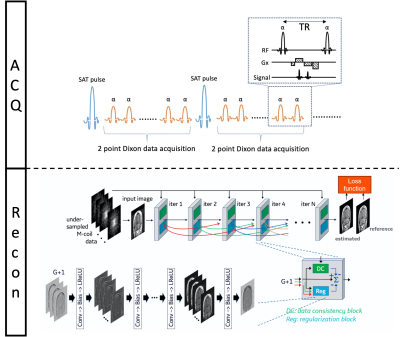
Figure.1. ACQ: Non-gated two-point Dixon SPGR based MRA pulse sequence. Intermittent SAT pulse placed on the FOV outside suppresses vein signal. Recon: Illustration of the architecture of DCI-Net with 2D convolution.
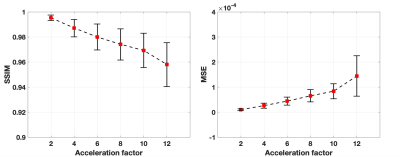
Figure.2. Calculated mean structure similarity (SSIM) index and mean-square error (MSE) on retrospectively undersampled data with different acceleration factors from full-sampled data of the reference standard. The error bar shows standard deviation across all the slices of FLEXA image reconstructed with the DCI-Net.
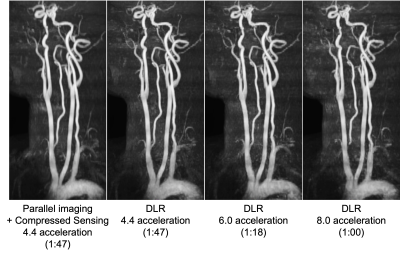
Figure.3. Representative DLR FLEXA images from prospectively undersampled data in comparison to the combined parallel imaging and compressed sensing. The DLR FLEXA shows comparable vessel delineation with a significant speedup.