0691
Detecting temperature-driven microstructural modulations in tissue using diffusion MRI1Center for Biomedical Imaging, Dept. Radiology, NYU Grossman School of Medicine, New York, NY, United States, 2Champalimaud Research, Champalimaud Centre for the Unknown, Lisbon, Portugal
Synopsis
Keywords: Diffusion/other diffusion imaging techniques, Microstructure
In this study, we uncover that the signal fraction of a fully restricted diffusion compartment in fixed white matter tissue, the so-called dot fraction, is dependent on the tissue temperature. In particular, we report a sudden increase in its nominal value of this fraction when the tissue temperature drops below approximately 32°C. The underlying mechanism remains unexplained, but its impact on modeling of ex vivo diffusion MRI cannot be underestimated. The observation corroborates our hypotheses of a temperature-dependent disruption of the multilamellar structure of the myelin sheath, leading to water trapped in vacuoles that are formed in the myelin sheath.Introduction
The workflow for MR imaging of fixed tissue is complex with many degrees of freedom [1]. The tissue temperature during MR scanning is one of the parameters in the workflow that is not always attended to. Several studies have indeed noted marked changes in tissue water pools upon changing from room temperature to body temperature [2,3]. We hypothesize that the tissue temperature might be an important confounding factor in the accuracy, specificity, and interpretation of biophysical models of diffusion. Endothermic phase transitions have been observed in the myelin of the central nervous system [4]. Previous studies reported on a reversible temperature-dependent disruption of the multilamellar structure of the myelin sheath and the formation of vacuoles at temperatures below 33°C [5]. While such phenomena have been observed in fresh tissue, it is not understood whether similar effect might confound fixed tissue. This concern might be particularly relevant for tissue that has been fixed with formaldehyde – while this fixation method results in a more favorable diffusion coefficient than glutaraldehyde, it may be prone to a suboptimal stabilization of the myelin sheath [6,7]. Here we propose to modulate temperature of perfusion-fixed white matter tissue and quantify the effect on biophysical modeling of diffusion MRI. In particular, we will tailor our experiments to the measurement of the so-called dot fraction, a signal compartment that represent fully restricted diffusion. While such compartment has previously been associated with microglial cells, its biophysical interpretation remains poorly understood. Ex vivo studies performed at room temperature report dot fractions up to 20% [8,9], while in vivo studies fail to observe a significant dot compartment in the brain white matter [10,11]. Therefore, we hypothesize that the dot fraction might in fact reflect to temperature-dependent disruption of the multilamellar structure of the myelin sheath.Methods:
All animal experiments followed European Directive 2010/63 and were preapproved by the Institution’s Review Board and the national competent authority.Tissue: We study the ex-vivo rat brain and focus on its Corpus Callosum as representative WM that was transcardially perfused using 4% paraformaldehyde following a conventional protocol [12]. The extracted brain was kept for 24h in 4% paraformaldehyde and then washed using PBS over two days (changed daily). The brain was then placed in a 10mm tube filled with Fluorinert for susceptibility matching.
MR protocol: One mid-sagittal slice of the sample was scanned on a 16.4T MR scanner (Bruker BioSpin) interfaced with a micro2.5 imaging probe with maximal gradient amplitude Gmax=1500mT/m and an AVANCE IIIHD console for tissue temperature control. We varied the temperature of the tissue gradually from 20°C to 40°C and back (See Figure 1). At each temperature, we acquired (1) Single-shell DWI data: for the estimation of the mean diffusivity (MD), (2) multi-echo spin echo for the estimation of $$$T_\mathrm{2}$$$, and (3) Single-direction ultra-high b-values in the direction parallel to the average fiber direction for the estimation of the fully restricted signal fraction. At 22°C and 37°C, we additionally acquired an extensive multi-shell diffusion MR protocol. All acquisitions were performed using the REMMI toolbox. All scan parameters are summarized in Figure 2.
MR analysis: The spatially localized fully restricted signal fraction $$$f^\bullet$$$ is computed as the signal estimated from the repeated (N=60) ultra-high b measurements in the direction parallel to the principal fiber direction using a Rician maximum likelihood estimator with pre-computed noise level normalized by the respective non-diffusion weighted signal. The MD is calculated from the diffusion tensors that are estimated voxel-wise by fitting the DTI model to the single shell data. The $$$T_\mathrm{2}$$$ is estimated by fitting a mono-exponential signal model to the multi-echo spin echo data. The parameters of a two-compartmental biophysical standard model are estimated from the multi-shell diffusion MR protocol [13].
Results
In figure 3, we show the fully restricted signal fraction $$$f^\bullet$$$, the MD, and $$$T_\mathrm{2}$$$ as a function of tissue temperature. We observe a linear trend of $$$T_\mathrm{2}$$$ and MD as a function of temperature. The fully restricted signal fraction – a proxy for the dot compartment - is highly dependent on the temperature, with a fast nominal increase when the temperature drops below approximately 32°C. Of note, all trends are reversible. In figure 4, we show the maps of the restricted signal fraction. The temperature-dependency of such restricted fraction impedes the accuracy of diffusion modeling – as shown in Figure 5.Discussion and Conclusion:
The effect of temperature and fixation on MD, inhomogeneous magnetization transfer, and relaxation times has been studied and recommendation for experimental design have been provided [1-3,6]. In this study, we uncover that the magnitude of the so-called “dot fraction” strongly depend on the temperature too. Of note, While the signal fraction of a fully restricted diffusion compartment is small – potentially even negligible at body temperature, we see a sudden increase in its nominal value when the tissue temperature drops below approximately 32°C. The underlying mechanism remains unexplained, but its impact on modeling of ex vivo diffusion MRI cannot be underestimated. However, we conclude that the observation corroborates our hypotheses of a temperature-dependent disruption of the multilamellar structure of the myelin sheath, leading to water trapped in vacuoles that are formed in the myelin sheath.Acknowledgements
All authors would like to thank Prof. Mark D Does (Vanderbilt University) for the remmiRARE sequence used in this study, supported by grant number NIH EB019980
Funding: LISBOA-01-0145-FEDER-022170, R01NS088040, P41EB017183, R01NS128190
References
[1] Schilling et al. arXiv:2209.13371(2022)
[2] Birkl et al. NMR in Biomedicine 29:458-465 (2016)
[3] Prevost et al. NeuroImage 236:118046 (2021)
[4] Moscarello et al. Biochimici et Biophysica Acta, 728:201-205 (1983)
[5] Mateu et al. J. Mol. Biol. 245, 110-125 (1995)
[6] Shepherd et al. MRM 62(1):26-34 (2009)
[7] Seifert et al. MRM 82:1504-1517 (2019)
[8] Stanisz et al. MRM 37:103-111 (1997)
[9] Alexander et al. NeuroImage 52:1374-1389 (2010)
[10] Tax et al. NeuroImage 210:116534 (2020)
[11] Dhital et al. NeuroImage 182:398-406 (2018)
[12] Veraart et al. eLife 9:e49855 (2020)
[13] Novikov et al. NeuroImage 174:518-538 (2018)
Figures
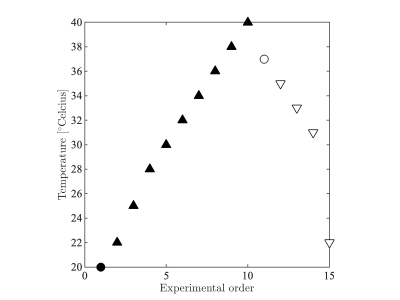
Figure 1: Tissue temperature throughout the experiments. At room temperature (20oC) and body temperature (37), an extensive multishell diffusion protocol was acquired in addition to the three faster protocols for the estimation of MD, $$$T_2$$$, and $$$f^\bullet$$$ . After each temperate change, the tissue temperate was stabilized during a two hour wait time..
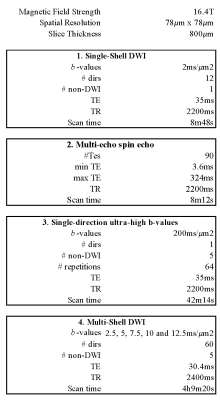
Figure 2: Scan parameters
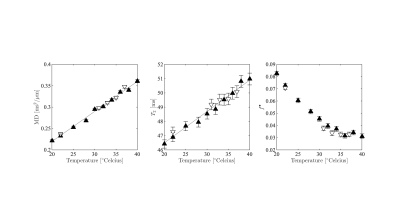
Figure 3: MD, $$$T_\mathrm{2}$$$, and $$$f^\bullet$$$ as a function of temperature. The ROI-averaged values and their standard error are shown. The color-encoding of the markers is in agreement with Figure 2 to differentiate between temperate increase and decrease.
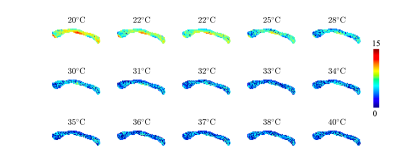
Figure 4: Maps of the fully restricted signal fraction $$$f^\bullet$$$ as a function of temperature.
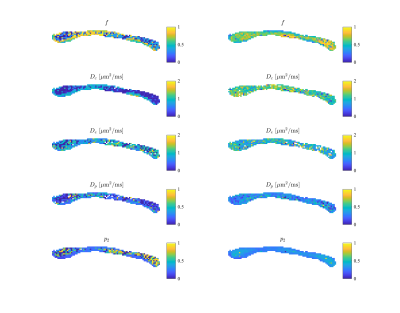
Figure 5: The model parameters of the biophysical standard model at 22°C (left) and 37°C (right) temperature. The parameters include intra-cellular signal fraction $$$f$$$, intra-cellular diffusivity $$$D_c$$$, parallel extra-cellular diffusivity $$$D_e$$$, perpendicular extra-diffusivity $$$D_p$$$, and dispersion index $$$p_2$$$.