0685
Quantifying human gray matter microstructure using NEXI and 300 mT/m gradients1Dept. of Radiology, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 2CUBRIC, Cardiff University, Cardiff, United Kingdom
Synopsis
Keywords: Microstructure, Modelling
For the first time, we report Neurite Exchange Imaging (NEXI) microstructure model parameters estimates in human cortex in vivo. We also investigate the performance of two extensions of this model, the addition of a dot compartment and a correction for wide pulses. Parameter estimates are consistent with previous findings in the rat cortex in vivo. Importantly, NEXI estimates displayed good scan-rescan reproducibility while retaining sensitivity to inter-subject differences. Future work will focus on improving the precision, in particular of the exchange time estimates, possibly leveraging multi-dimensional diffusion MRI acquisitions.
Background
NEXI1 (or SMEX2) is a gray matter (GM) two-compartment diffusion model that accounts for inter-compartment exchange. It has first been implemented in a preclinical setting in vivo1 and ex vivo2. Here, we aimed to test the performance of NEXI in the human cortex in vivo, exploiting the Connectom strong gradient system to achieve short diffusion times and high b-values. We report NEXI estimates across various GM ROIs based on three implementations: the 2-compartment model in the narrow pulse approximation (NEXI), the two-compartment model accounting for gradient pulse duration2 (NEXI finite pulses, or NEXI_fp), and a three-compartment extension, allowing for an extra parameter2 capturing a possible Rician bias or ‘dot’ compartment (NEXI_dot). We also compare the NEXI estimates of inter-compartment exchange time tex with the one from the Kärger model time-dependent kurtosis1,3 . Finally, we test the reliability of NEXI estimates by comparing intra-subject (scan-rescan) to inter-subject variability.Methods
Four healthy volunteers were scanned (3 of whom rescanned on a different day). Acquisition: An MPRAGE was acquired for anatomical reference (1-mm isotropic resolution). Diffusion-weighted images were acquired on a 3T Siemens Connectom system using a PGSE EPI sequence with combinations of b-values of 1 (13 directions), 2.5 (25 dir.), 4 (25 dir.), 6 (32 dir.) and 7.5ms/µm² (65 dir.), and Δ=20, 29, 39 and 49ms; δ=9ms, in addition to 15 b=0 images per Δ, at 1.8-mm isotropic resolution, TE/TR=76/ 3700 ms, total scan time: 45min. Processing: Multi-shell multi-diffusion time data was preprocessed jointly; steps included MP-PCA magnitude denoising4, Gibbs ringing correction5, distortion and eddy current correction6. DKI tensors were calculated to extract Mean Diffusivity (MD) and Mean Kurtosis (MK) for each diffusion time, using b-values up to 2.5ms/µm². was then fit to MK to yield an estimation of 1,3. The grey matter region of interests (ROIs) from the Desikan-Killiany-Tourville atlas7 were segmented on the MPRAGE image using FastSurfer8 and projected onto the diffusion native space using linear registration9 of b=0 images to MPRAGE images. The ROI-averaged powder-averaged signals were then extracted to fit the three model implementations: NEXI, NEXI_fp and NEXI_dot. The models were fit using non-linear least squares, initialized following a grid search, and yielding estimates for tex, the intra and extra-neurite apparent diffusivities Di and De and the intra-neurite signal fraction f, as well as the potential ‘dot’ compartment signal fraction fdot. NEXI ROI estimates were then compared between scan-rescan and across subjects.Results and discussion
MD was almost independent of the diffusion time, with a slope of -7.5x10-4 µm²/ms², and MK decreased more markedly with time (Fig. 1A), consistent with previous studies1,10 and with a regime dominated by exchange rather than structural disorder. The estimated was ~30 ms, (Fig. 1B) but with a broad distribution across the cortex. NEXI parametric maps were consistent with expected neuroanatomy in the human brain (Fig. 2). However, Di was the most challenging parameter to estimate and often hit the upper bound of 3.5 ms/µm². NEXI estimates calculated across GM ROIs (Fig. 3A) were consistent with previous studies of rat cortex in vivo1, with however longer tex (median 87 ms). NEXI_fp resulted in shorter tex estimates (median 19 ms, more consistent with rat cortex in vivo) and higher f (0.53), possibly a signature of more myelinated and denser human cortex vs rat, or more partial volume with WM. NEXI_dot reduced the tex estimate further, while changing compartment diffusivities completely. The corrected Akaike information criterion (AICc) attributed poorest fitting performance to NEXI_dot, followed by NEXI_fp, and NEXI as best (Fig. 3A-B). Broad interquartile intervals overall suggest nonetheless substantial impact from partial volume with CSF and/or WM, or GM microstructure variability across the cortex. Distributions of NEXI estimates across the cortex display good inter-subject consistency (Fig. 4A) and scan-rescan reproducibility (Fig. 4B), also shown in the ROI analysis (Fig. 5), with the mean inter-subject confidence interval being larger than the mean scan-rescan confidence intervals by a factor of 1.8 for tex, 1.5 for Di, 1.3 for De and 2.3 for f. The lower intra-subject vs inter-subject variability suggests NEXI is reproducible while sensitive to individual differences, notably with f (Figs. 4-5). Largest inter-subject variability is recorded for tex and f, the former likely due to fit uncertainty but the latter possibly due to genuine individual differences, which remain to be confirmed.Conclusion
For the first time, we report NEXI model parameters estimates in human cortex in vivo. The estimated neurite fraction and compartment diffusivities are consistent with previous findings in the rat cortex in vivo, while the exchange time is longer. Our data do not favor the addition of a dot compartment to the NEXI model of two exchanging compartments. The correction for wide pulses NEXI_fp however brings tex estimates closer to the ones initially reported in the rat cortex (albeit with higher f), but results in poorer fit quality, which requires further investigation. Notably, the typical exchange time is in all cases longer (20 – 90ms) than reported in a previous similar study11. Importantly, NEXI estimates displayed good scan-rescan reproducibility while retaining sensitivity to inter-subject differences. Di and tex remain the most challenging parameter to estimate. Future efforts will focus on possible improvements, such as more advanced acquisition schemes.Acknowledgements
The authors thank Sune Jespersen and Jonas Olesen for sharing the NEXI_fp code. QU, TP and IJ are supported by SNSF Eccellenza grant PCEFP2_194260. MP is supported by UKRI Future Leaders Fellowship MR/T020296/2. The data were acquired at the UK National Facility for In Vivo MR Imaging of Human Tissue Microstructure funded by the EPSRC (grant EP/M029778/1), and The Wolfson Foundation. DKJ is supported in part by a Wellcome Trust Investigator Award (096646/Z/11/Z) and Wellcome Trust Strategic Award (104943/Z/14/Z).
References
[1] Jelescu, NeuroImage 2022 [2] Olesen, NeuroImage 2022 [3] Jensen, NMR in Biomed 2010 [4] Veerart, NeuroImage 2016 [5] Kellner, MRM 2016 [6] Andersson, NeuroImage 2016 [7] Klein and Tourville, Frontiers in Neuroscience 2012 [8] Henschel, NeuroImage 2020 [9] Avants et al., Insight j, 2009, [10] Lee, NeuroImage 2020 [11] Lee, ISMRM Diffusion Day 2021.
Figures
Figure 1. A. Time-dependent MD and MK in the cortex, averaged over the four subjects. B. Distribution of estimates across the cortex voxels.
Figure 2. Axial slice of NEXI parametric maps in one subject. tex and De are consistent throughout the cortex, but tex is presumably longer in the WM and cannot be reliably estimated using available diffusion times. f displays the expected anatomical pattern in white vs gray matter. Di shows large variability across voxels, while hitting its upper bound frequently.
Figure 3. A. Median and quartiles of NEXI estimations across all GM ROIs and subjects. Due to the small number of ROIs, the estimates that hit a bound are kept. Akaike Information Criterion (AICc) is given for each model (NEXI, NEXI_fp and NEXI_dot). B. Comparisons between the signal in the right posterior cingulate (dots) and the fitted signal from each model at different diffusion times. The signal decreases with longer times, as expected from exchange, but the quality of fit is variable.
Figure 4. Distributions of voxel-wise NEXI estimates across the cortex of the 4 subjects (A) and between two scans of the same subject (B) (mean: solid line, confidence interval: shaded area around the mean). Inter-subject variability is higher than intra-subject variability, particularly for Di and f. The distributions are also multi-peaked for f and Di, which shows different NEXI solutions may be optimal for various cortical areas.
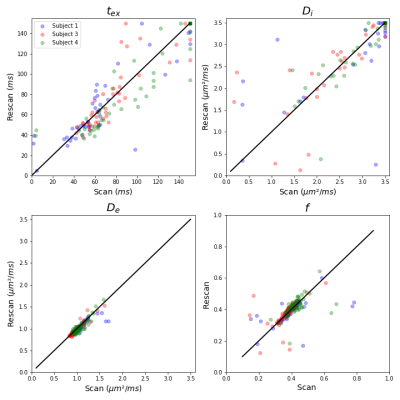
Figure 5. Comparison between scan and rescan of the NEXI parameters fitted in each GM ROI, of the three subjects who had a second scan. The estimated values of De and f are consistent from scan to rescan, and in particular, f allows seeing differences between subjects each forming a small cluster. The difficulty of estimating tex and Di results in a scatter with a high variance around the identity, in black, and in several ROIs hitting the upper bound of Di in one of the scans.