0677
Evaluating Echo Planar Spectroscopic Imaging with a Columnar Excitation for "Virtual Biopsies"
Michael S Yao1,2, Andrew Van3,4, James Gee2, Murray Grossman5, David J Irwin5,6, and M Dylan Tisdall2
1Department of Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 2Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 3Department of Neurology, Washington University School of Medicine, St. Louis, MO, United States, 4Department of Biomedical Engineering, Washington University School of Medicine, St. Louis, MO, United States, 5Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 6Pathology and Laboratory Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
1Department of Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 2Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 3Department of Neurology, Washington University School of Medicine, St. Louis, MO, United States, 4Department of Biomedical Engineering, Washington University School of Medicine, St. Louis, MO, United States, 5Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 6Pathology and Laboratory Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Keywords: Quantitative Imaging, Quantitative Imaging
The acquisition of high-resolution quantitative measurements is of particular interest in studying the laminar structure and layer-specific pathology in the cerebral cortex. In this work, we propose a method to address this need by acquiring 1-D echo planar spectroscopic imaging (EPSI) as a "virtual biopsy" with 200 µm resolution along the readout direction. Our sequence yields expected spectra for common compounds and produces high-quality quantitative T2* and off-resonance measurements in ex vivo brain tissue. Our goal is to use this quantitative virtual biopsy to discriminate laminar variations in cortical iron deposition in diseases such as frontotemporal lobar degeneration (FTLD).Introduction
Recent advancements in neuroimaging have demonstrated the use of columnar excitations and high-resolution 1-D readouts to encode cortical laminar features1. Prior work has focused on columnar diffusion-weighted imaging (DWI), which is strongly sensitive to the laminar features of the cortical myeloarchitecture. However, neurodegenerative diseases—in particular Alzheimer's Disease (AD)2, Amyotrophic lateral sclerosis (ALS)3, and frontotemporal lobar degeneration (FTLD)4—also produce notable iron deposition within the cortical laminae. The layer-specific resonance frequency shift that results from the cortical distribution of laminar iron has previously been imaged using 2-D T2*-weighted3,5 and 2-D phase-contrast methods6. Combing these concepts, we explored the use of columnar excitations and high-resolution 1-D echo planar spectroscopic imaging (EPSI) readouts7 to produce a "virtual biopsy" with depth-resolved quantification of the water signal's off-resonance shift, towards the goal of resolving the pathologic iron distributions associated with different neurodegenerative diseases that can be difficult to distinguish in vivo2-4.Methods
A spin-echo EPI sequence was modified to achieve columnar excitation and 1-D EPSI readout (Fig. 1). The refocusing RF pulse was adjusted to be applied in the phase-encoding (PE) direction orthogonal to the excitation pulse used for slice-selection. In this way, coherent signal is only acquired from a 1-D columnar segment at the intersection of the excitation and refocusing slice-selection planes. Removing the PE blips from the EPI readout train and centering the first readout of the train at the spin echo produces a 1-D EPSI, which we use to acquire multiple sequential echoes from this "virtual biopsy" column. To achieve sufficient SNR, the experiment is then repeated to acquire multiple averages.To demonstrate our proposed technique, we imaged a phantom constructed using four 50-mL conical tubes individually filled with methanol (MeOH), ethanol (EtOH), olive oil, and water on a 3T scanner (Prisma, Siemens Healthineers, Erlangen, Germany) using the vendor's 64-channel head coil (with 52 channels active). We collected 32 averages of the virtual biopsy scan with 200 µm resolution and 150 mm FOV along the readout dimension, and 3.0 mm thickness for both the excitation and refocusing pulses. Additional parameters were 16 ms TE, 1000 ms TR, 304 Hz/px bandwidth, 3.92 ms echo-spacing, and 36 sec total scan time.
To explore whether our EPSI sequence could interrogate clinical pathology, we also imaged a formalin-fixed ex vivo cerebral human hemisphere specimen. The sample donor had pathologically confirmed progressive supranuclear palsy (PSP), which we have previously shown to have focal cortical iron deposition observable in the motor cortex on T2*-weighted ex vivo scans4,8. We used the same 3T imaging setup with similar imaging parameters, collecting 32 averages using 48 active channels with 200 µm resolution; 140 mm readout FOV; 3.0 mm slice thickness; 15 ms, 30 ms, and 45 ms TE; 1000 ms TR; 388 Hz/px bandwidth; 3.22 ms echo-spacing; and 36 sec total scan time.
We phase-corrected for EPI even-odd readout coherence by computing the all-pass delay filter that optimally aligns even- and odd-polarity readouts9, and combined individual coil data using the approach described by Hu et al (2021)10. To analyze our ex vivo data, we calculated the spatially dependent peak off-resonance and both T2* and T2 relaxation time using methods described by Funai et al (2008)11 and Bonny et al (1996)12, respectively. The code and data for our work is available at https://www.github.com/michael-s-yao/VirtualBiopsy and is licensed under the MIT License.
Results and Discussion
Figure 2 illustrates our proposed EPSI method using a phantom consisting of methanol, ethanol, olive oil, and water. Our imaging bandwidth was approximately 2 ppm, which led to aliasing of resonance peaks such as the hydroxyl group contribution in the alcohol spectra that fall outside of our limited bandwidth. However, for practical clinical applications, resolving T2* and off-resonance shifts in human tissue are well-known to be resolvable within our 2 ppm bandwidth2-4.To further validate this claim, we imaged the motor cortex of an ex vivo specimen demonstrating the pathophysiology of PSP to explore laminar cortical iron deposition using our EPSI approach (Fig. 3). As expected, the white matter regions at approximately 65 and 72 mm along the biopsy dimension immediately surrounding the central sulcus are characterized by shorter T2* and T2 relaxation times due to myelin and co-located iron in the white matter. A "dip" in the T2* within the cortex at roughly 67.5 mm is likely due to pathologic iron deposits in the middle layers of the cortex, as previously described for this disease4. Detecting variations in relaxation times, peak off-resonances, and other features that vary with cortical anatomy is made possible by our high spatial resolution of 200 µm along the biopsy direction.
Conclusion
We have presented a method for efficient virtual biopsy acquisition via EPSI imaging for high-resolution tissue resonance spectra. Our proposed technique trades a second spatial dimension for frequency information along the readout dimension, which may enable future studies on early detection of FTLD and other neuropathological diseases through noninvasive cortical iron analysis4. Further work remains to be done to achieve this goal, such as in motion correction and phase stabilization that are necessary for real-world in vivo applications.Acknowledgements
This work was funded by NIH awards P01AG066597, R01NS109260, P30AG072979, and R01AG054519; the Penn Institute on Aging; the Wyncote Foundation; and The DeCrane Family Fund for Primary Progressive Aphasia Research.References
1. Balasubramanian M, Mulkern RV, Neil JJ, Maier SE, Polimeni JR. Probing in vivo cortical myeloarchitecture in humans via line-scan diffusion acquisitions at 7T with 250-500 micron radial resolution. Mag Reson Med. 2021;85(1):390-403. doi: 10.1002/mrm.284192. van Duijn S, Bulk M, van Duinen SG, Nabuurs RJA, van Buchem MA, van der Weerd L, Natté R. Cortical iron reflects severity of Alzheimer's disease. J Alzheimer's Disease. 2017;60:1533-45. doi: 10.3233/JAD-161143
3. Kwan JY, Jeong SY, Van Gelderen P, Deng HX, Quezado MM, Danielan LE, Butman JA, Chen L, Bayat E, Russell J, Siddique T, Duyn JH, Rouault TA, Floeter MK. Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 Tesla MRI and pathology. PLoS ONE. 2012;7(4):e35241. doi: 10.1371/journal.pone.0035241
4. Tisdall MD, Ohm DT, Lobrovich R, et al. Ex vivo MRI and histopathology detect novel iron-rich cortical inflammation in frontotemporal lobar degeneration with tau versus TDP-43 pathology. Neuroimage Clin. 2022;33:102913. doi: 10.1016/j.nicl.2021.102913
5. Kenkhuis B, Jonkman LE, Bulk M, Buijs M, Boon BDC, Bouwman FH, Geurts JJG, van de Berg WDJ, van de Weerd, L. 7T MRI allows detection of disturbed cortical lamination of the medial temporal lobe in patients with Alzheimer's disease. Neuroimage Clin. 2019;21:101665. doi: 10.1016/j.nicl.2019.101665
6. Duyn JH, van Gelderen P, Li TQ, de Zwart JA, Koretsky AP, Fukunaga M. High-field MRI of brain cortical substructure based on signal phase. Proc Nat Acad Sci. 2007;104(28): 11796-801. doi: 10.1073/pnas.0610821104
7. Mansfield P. Spatial mapping of the chemical shift in NMR. Mag Reson Med. 1984;1(3):370-86. doi: 10.1002/mrm.1910010308
8. Kovacs GG, Lukic MJ, Irwin DJ, Arzberger T, Respondek G, Lee EB, Coughlin D, Giese A, Grossman M, Kurz C, McMillan CT, Gelpi E, Compta Y, van Swieten JC, Laat LD, Troakes C, Al-Sarraj S, Robinson JL, Roeber S, Xie SX, Lee VY, Trojanowski JQ, Höglinger GU. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathologica. 2020;140(2):99–119. doi: 10.1007/s00401-020-02158-2
9. Tisdall MD. Scrambled readout polarities in 3D-encoded EPI markedly reduce the coherence of N/2 ghosts. Proc Intl Soc Mag Reson Med. 2020;28(3420).
10. Hu W, Liu H, Chen D, Qui T, Sun H, Xiong C, Lin J, Guo D, Chen H, Qu X. Coil combination of multichannel single voxel magnetic resonance spectroscopy with repeatedly sampled in vivo data. Molecules. 2021;26(13):3896. doi: 10.3390/molecules26133896
11. Funai AK, Fessler JA, Yeo DTB, Olafsson VT, Noll DC. Regularized field map estimation in MRI. IEEE Trans Med Imaging. 2008;27(10):1484-94. doi: 10.1109/TMI.2008.923956
12. Bonny JM, Zanca M, Boire JY, Veyre A. T2 maximum likelihood estimation from multiple spin-echo magnitude images. Mag Reson Med. 1996;36:287-93. doi: 10.1002/mrm.1910360216
Figures
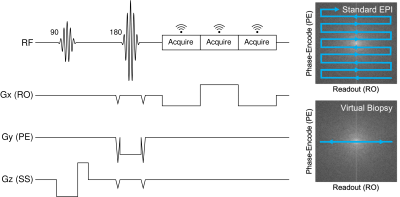
(Left) MR pulse sequence diagram for our EPSI-like virtual biopsy sequence. (Right) Visual illustration of our virtual biopsy k-space trajectory compared to that of typical EPI imaging approaches.
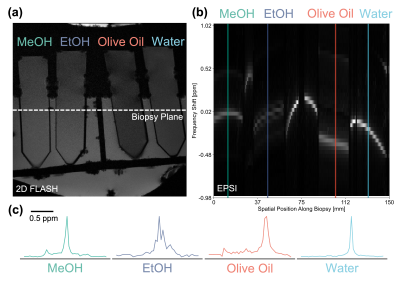
EPSI spectroscopic analysis of a phantom containing four chemical samples: methanol (MeOH), ethanol (EtOH), olive oil, and water. (a) Reference 2-D FLASH scan of the phantom setup indicating the virtual biopsy readout axis. (b) EPSI virtual biopsy T2-weighted spectrum. (c) Plotting the 1-D MR spectra at the four indicated locations demonstrate the expected peaks for each of the imaged compounds.
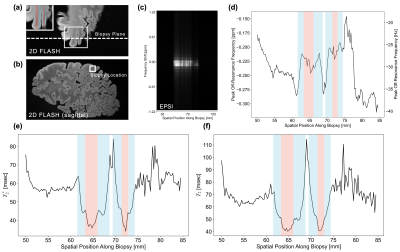
EPSI imaging of an ex vivo specimen of a cortical hemisphere from a patient with PSP. Reference (a) 2-D FLASH scan co-planar with our 1-D EPSI (approximately coronal/sagittal) and (b) sagittal 2-D FLASH scans demonstrate the virtual biopsy readout axis and biopsy location, respectively. (c) EPSI virtual biopsy T2-weighted spectrum using a TE of 15 ms. (d) Peak off-resonance frequency, (e) T2*, and (f) T2 relaxation times as a function of spatial position in the virtual biopsy. Cortical regions are highlighted in blue and white matter are highlighted in red.
DOI: https://doi.org/10.58530/2023/0677