0673
Influence of gadolinium, field-strength and sequence on quantified perfusion values in phase-resolved functional lung MRI1Institute for Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany, 2Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), Member of the German Centre for Lung Research (DZL), Hannover, Germany, 3MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Keywords: Quantitative Imaging, Perfusion, Lung
The aim of this study is the evaluation of the influences of previously applied gadolinium-based contrast media, differing field strength and differing imaging sequence on the lately proposed perfusion quantification for PREFUL MRI. The results indicate severe alterations and distortions of the quantified perfusion parameters after gadolinium administration and depending on the field strength and on the applied sequence type. The results show, that future multicenter studies need to strictly adhere to an identical imaging protocol using the same field strengths to generate comparable results across all sites.Introduction
Functional lung imaging has proven to offer valuable information for surveillance and early detection of various pulmonary diseases (1–4). Techniques like phase-resolved functional lung (PREFUL) MRI (5) avoid potential risks of contrast media, increase patient comfort and are already implemented in the clinical routine (6–9). Recently, a perfusion quantification technique for PREFUL MRI was developed and showed physiologically plausible results and good agreement to dynamic contrast-enhanced MRI in patients with chronic obstructive pulmonary disease (COPD) and chronic thromboembolic pulmonary hypertension (CTEPH) (10). However, a major challenge of medical imaging is the limited comparability across different sites, protocols and software. These influences on the MRI signal are highly complex, which challenges absolute quantification of functional parameters as they are based on stable reference signals (11,12). Therefore, the aim of this study is the evaluation of the influences of previously applied gadolinium-based contrast media, a differing field strength and a differing imaging sequence on the lately proposed perfusion quantification for PREFUL MRI.Methods
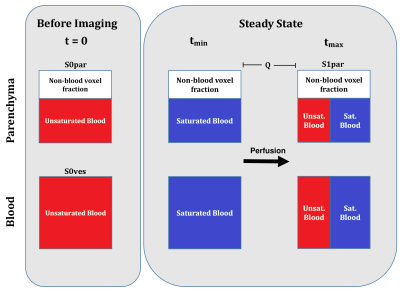
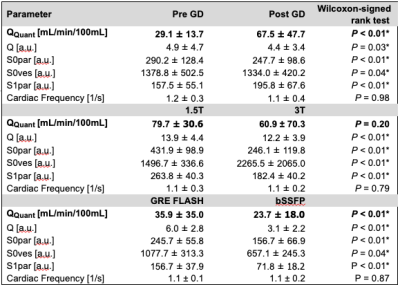
In this study, data sets of 48 participants from three cohorts were evaluated. All datasets were postprocessed and analyzed retrospectively. The first cohort (gadolinium cohort) was imaged before and after the administration of gadolinium-based contrast media. The second cohort (field strength cohort) was imaged at 1.5 Tesla (T) and 3T scanners. The third cohort (sequence cohort) was imaged with a FLASH and with a bSSFP sequence. The corresponding series in each cohort were registered to allow for voxel wise comparison. Perfusion was quantified (QQuant) by estimating the proton density and considering the median height of the signal decay towards the steady-state (10). For this, the signals at several time points (t) of the time series were extracted to estimate the proportion of spins being exchanged per heart beat (exchange fraction) and the proportion of blood per voxel (blood fraction), which were combined together with the time interval between two heartbeats (T) and additional scaling factors (Figure 1).Results
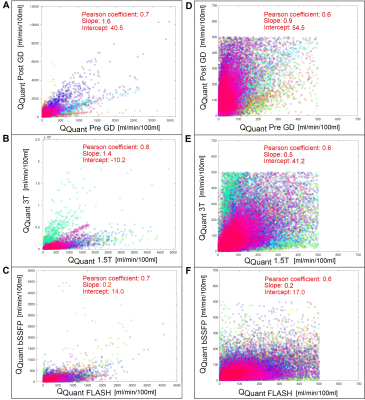
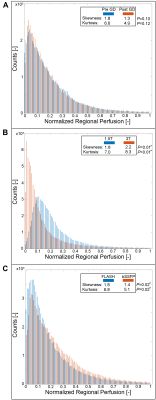
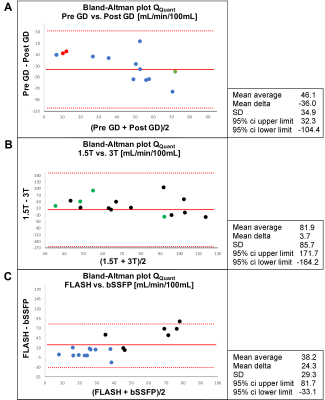
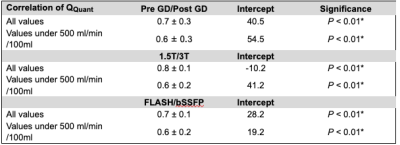
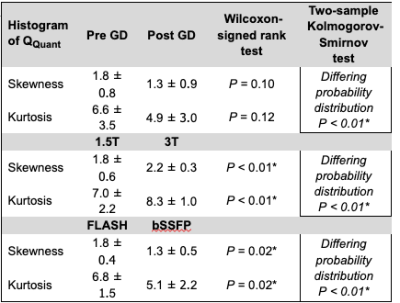
The heart frequency presented no significant change between the corresponding series in any cohort (P ≥ 0.79) (Table 1). Voxel wise Pearson correlation of QQuant between both series was highly significant with a correlation coefficient of ≥ 0.7 in all cohorts (Figure 2 and Table 2). However, the two-sample Kolmogorov-Smirnov test revealed a differing probability distribution of the histogram values in all cohorts (Figure 3 and Table 3). In the gadolinium cohort, the quantified perfusion increased significantly (Figure 4). No major change was detected for the value distribution in the normalized histogram analysis with skewness from 1.8 to 1.3 (P = 0.10) and kurtosis from 6.6 to 4.9 (P = 0.12). In the field-strength cohort, no significant change of the quantified perfusion was shown (P = 0.20). The skewness and kurtosis increased significantly from 1.8 to 2.2 and from 7.0 to 8.3. In the sequence cohort, the quantified perfusion decreased significantly from 35.9 using FLASH to 23.7 ml/min/100ml using bSSFP. The skewness and kurtosis decreased significantly from 1.8 to 1.4 and from 6.8 to 5.1.Discussion
Significant alterations of the median values and the value distribution were detected in each cohort. The different slopes and spread of extremely high values in Figure 2 representing central vessels, might be the result of individual anatomy, flow velocities and heart function between or could display a systematic error. Nevertheless, all cohorts presented markedly deviations of the slope and high intercepts when correlating both corresponding series. Regarding the value distribution of the quantified parameter QQuant, only the gadolinium cohort delivered no significant change of the skewness and kurtosis. This is likely due to the use of the identical sequence and field strength for both series. In the field strength cohort, the interquartile range presented a markedly increase at 3T for all parameters, especially for the vessel signal at the beginning of the sequence (S0ves). Likely, the increasing spread of the values is caused by increasing field inhomogeneities occurring in lung MRI on interfaces like vessel walls at increasing B0 (13). The observed signal reduction within the parenchyma (S0par and S1par) at 3T could be the result of increasing susceptibility artifacts and spin-spin interactions shortening the T2* relaxation time, while the signal in the vessel (S0ves) is increasing due to the gain of the SNR (13–16). The sequence parameters of the bSSFP could be further optimized to increase the overall lung signal and its variation over time (17,18). The high sensitivity of the bSSFP sequence to flow effects might lead to fewer zero values within the perfusion maps causing lower skewness and kurtosis in the histogram analysis. A limitation of our study are the differing sequence parameters between 1.5T and 3T. Additionally, the different patient groups could be affected differently, but the limited number and heterogeneous group of patients hinders a subgroup analysis. The results show, that future multicenter studies need to strictly adhere to an identical imaging protocol using the same field strengths to generate comparable results across all sites. The results indicate severe alterations and distortions of the quantified perfusion parameters after gadolinium administration and depending on the field strength and on the applied sequence type.Acknowledgements
This work was funded by the German Center for Lung Research (DZL). The authors would like to express their gratitude to the radiographers from the Department of Radiology for their support with the MR measurements and patient care.References
1. Edelman RR, Hatabu H, Tadamura E, Li W, Prasad P V. Noninvasive assessment of regional ventilation in the human lung using oxygen–enhanced magnetic resonance imaging. Nat. Med. 1996;2:1236–1239 doi: 10.1038/nm1196-1236.
2. Berthezène Y, Vexler V, Clément O, Mühler A, Moseley ME, Brasch RC. Contrast-enhanced MR imaging of the lung: assessments of ventilation and perfusion. Radiology 1992;183:667–672 doi: 10.1148/radiology.183.3.1584916.
3. Roberts DA, Gefter WB, Hirsch JA, et al. Pulmonary Perfusion: Respiratory-triggered Three-dimensional MR Imaging with Arterial Spin Tagging—Preliminary Results in Healthy Volunteers. Radiology 1999;212:890–895 doi: 10.1148/radiology.212.3.r99se35890.
4. Wielpütz MO, von Stackelberg O, Stahl M, et al. Multicentre standardisation of chest MRI as radiation-free outcome measure of lung disease in young children with cystic fibrosis. J. Cyst. Fibros. 2018;17:518–527 doi: 10.1016/j.jcf.2018.05.003.
5. Voskrebenzev A, Gutberlet M, Klimeš F, et al. Feasibility of quantitative regional ventilation and perfusion mapping with phase‐resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients. Magn. Reson. Med. 2018;79:2306–2314 doi: 10.1002/mrm.26893.
6. Klimeš F, Voskrebenzev A, Gutberlet M, et al. 3D phase‐resolved functional lung ventilation MR imaging in healthy volunteers and patients with chronic pulmonary disease. Magn. Reson. Med. 2021;85:912–925 doi: 10.1002/mrm.28482.
7. Kaireit TF, Kern A, Voskrebenzev A, et al. Flow Volume Loop and Regional Ventilation Assessment Using Phase‐Resolved Functional Lung ( PREFUL ) MRI : Comparison With 129 Xenon Ventilation MRI and Lung Function Testing. J. Magn. Reson. Imaging 2021;53:1092–1105 doi: 10.1002/jmri.27452.
8. Voskrebenzev A, Kaireit TF, Klimeš F, et al. PREFUL MRI Depicts Dual Bronchodilator Changes in COPD: A Retrospective Analysis of a Randomized Controlled Trial. Radiol. Cardiothorac. Imaging 2022;4 doi: 10.1148/ryct.210147.
9. Zanette B, Schrauben EM, Munidasa S, et al. Clinical Feasibility of Structural and Functional MRI in Free‐Breathing Neonates and Infants. J. Magn. Reson. Imaging 2022;55:1696–1707 doi: 10.1002/jmri.28165.
10. Glandorf J, Klimeš F, Behrendt L, et al. Perfusion quantification using voxel-wise proton density and median signal decay in PREFUL MRI. Magn. Reson. Med. 2021;86:1482–1493 doi: 10.1002/mrm.28787.
11. Bloem JL, Reijnierse M, Huizinga TWJ, van der Helm-van Mil AHM. MR signal intensity: staying on the bright side in MR image interpretation. RMD Open 2018;4:e000728 doi: 10.1136/rmdopen-2018-000728.
12. Gulani V, Seiberlich N. Quantitative MRI: Rationale and Challenges. In: ; 2020. pp. xxxvii–li. doi: 10.1016/B978-0-12-817057-1.00001-9.
13. Soher BJ, Dale BM, Merkle EM. A Review of MR Physics: 3T versus 1.5T. Magn. Reson. Imaging Clin. N. Am. 2007;15:277–290 doi: 10.1016/j.mric.2007.06.002.
14. Glandorf J, Klimeš F, Voskrebenzev A, et al. Comparison of phase-resolved functional lung (PREFUL) MRI derived perfusion and ventilation parameters at 1.5T and 3T in healthy volunteers. PLoS One 2020;15:1–14 doi: 10.1371/journal.pone.0244638.
15. Gai ND, Malayeri AA, Bluemke DA. Three-dimensional T1 and T2* mapping of human lung parenchyma using interleaved saturation recovery with dual echo ultrashort echo time imaging (ITSR-DUTE). J. Magn. Reson. Imaging 2017;45:1097–1104 doi: 10.1002/jmri.25487.
16. Hatabu H, Alsop DC, Listerud J, Bonnet M, Gefter WB. T2* and proton density measurement of normal human lung parenchyma using submillisecond echo time gradient echo magnetic resonance imaging. Eur. J. Radiol. 1999;29:245–52 doi: 10.1016/S0720-048X(98)00169-7.
17. Bauman G, Pusterla O, Bieri O. Ultra-fast Steady-State Free Precession Pulse Sequence for Fourier Decomposition Pulmonary MRI. Magn. Reson. Med. 2016;75:1647–1653 doi: 10.1002/mrm.25697.
18. Bauman G, Pusterla O, Bieri O. Functional lung imaging with transient spoiled gradient echo. Magn. Reson. Med. 2019;81:1915–1923 doi: 10.1002/mrm.27535.
Figures