0668
Accurate B1 Mapping with Actual Flip Angle Imaging (AFI) in the Presence of Fat1Radiology, University of Wisconsin, Middleton, WI, United States, 2Radiology, University of Wisconsin, Madison, WI, United States, 3University of Washington, Seattle, WA, United States
Synopsis
Keywords: Quantitative Imaging, Artifacts, B1 mapping; quantitative; parameter mapping
Actual Flip angle Imaging (AFI) is an efficient B1 mapping method requiring the proper spoiling of transverse magnetization. Optimal spoiling can be achieved using large spoiling gradients enabling water diffusion-based signal decay. However, spoiling the non-aqueous signal like from fat is typically ignored in AFI optimizations. We demonstrate that infinitesimal diffusion in the fat signal makes fat spoiling in AFI unachievable in the reasonable scan time and that incomplete fat spoiling is a major source of previously unexplained AFI errors. We propose method to minimize them using a superposition model of the spoiling artifacts and chemical shift encoded fat/water separation.Introduction
Actual Flip angle Imaging (AFI) is an efficient $$$B1$$$ mapping method, which requires proper spoiling of transverse magnetization [1]. Optimal spoiling can be achieved in the presence of large spoiling gradients enabling water diffusion-based signal decay [2]. This approach provides high $$$B1$$$ mapping accuracy in tissues of diagnostic interest due to sufficiently high diffusion coefficients in them [2]. Simultaneously, spoiling the non-aqueous signal, i.e. from fat, is typically ignored in AFI optimizations, due to fat not being a primary imaging target in many standard quantitative MRI approaches, especially in neuroimaging. Here, we argue that infinitesimal diffusion in the fat makes fat spoiling in AFI unachievable in a reasonable scan time. We demonstrate that incomplete fat spoiling is a major source of previously unexplained AFI errors affecting not only fatty but also other tissues. Finally, we propose a method to minimize these errors using a superposition model of the spoiling artifacts and chemical shift encoded (CSE) fat/water separation.Theory
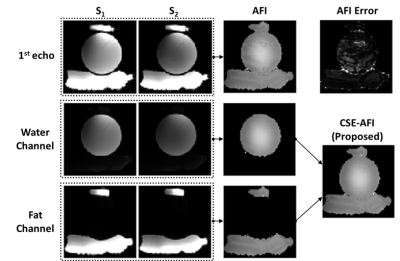
AFI sequence collects the signals $$$S_1$$$ and $$$S_2$$$ from two alternating spoiled gradient echo (SPGR) intervals with repetition times $$$\rm{TR}_1$$$ and $$$\rm{TR}_2$$$ ($$$\rm{TR}_2/\rm{TR}_1=n,n\approx4-5$$$ [2]), with the areas of spoilers $$$G_1,G_2$$$ at the end of each $$$TR$$$ related as $$$A_{G_2}=n\cdot{A_{G_1}}$$$. The effect of diffusion on the spoiling can be estimated based on the published data using diffusion dampening approach [3]. The dampening coefficient $$$d=D\,b$$$ depends linearly on the diffusion coefficient $$$D$$$ and quadratically on area of the gradient spoilers: $$b=\left(\gamma\int_0^{\rm TR_1}{\bf G}(\tau)d\tau\right)^2\cdot\rm{TR}_1.$$ Following results of [3], the tissue-dependence of optimal RF spoiling can be ignored if $$$d\ge1$$$. For example, for a typical range of tissue water diffusion coefficients, assuming gradient strength 28 mT/m, this condition leads to attainable 16ms $$$G_1$$$ duration [3]. However, the diffusion of fatty acids and triglycerides is about two orders of magnitude smaller than that of water [4], requiring the duration to increase by an order of magnitude, thus making it infeasible to implement without violating the scan time limits and AFI assumption ($$$\rm{TR}_1,\rm{TR}_2\ll T1$$$). Therefore, the operation within the feasible spoiling regimen would result in a situation where the fat signal is partially spoiled, and the associated breakdown of steady-state condition may lead not only to localized but also to distributed errors, typically appearing as ghost signals [5]. Following the linearity of the signal formation, we note that the fat ghosts retain the distinct chemical shift signature of fat. Therefore, we hypothesize that they can be removed from source images prior to AFI processing by separating AFI images into fat and water channels using CSE F/W separation methods provided that multiple echoes are collected in AFI.Methods
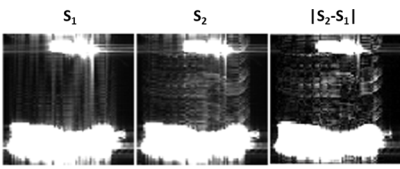
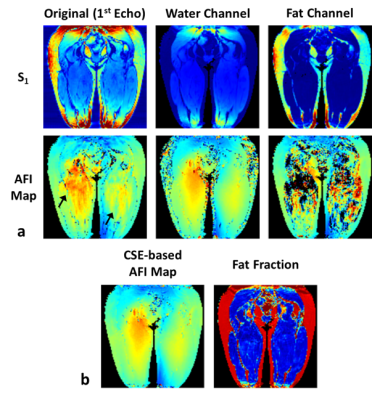
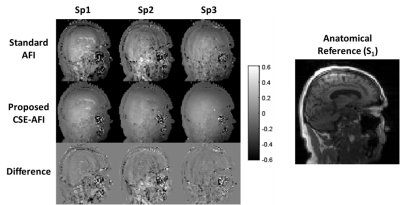
Experiments were performed on a 3.0T GE Discovery MR750 (Waukesha, WI). AFI was implemented with four-echo readout [6]. AFI spoiling was implemented using RF phase increments and gradient spoilers identified, according to [2], as standard, strong, and complete spoiling regimes for water-containing tissues. The phantom experiments were performed in objects comprising a plastic sphere either empty or filled with Gd-dopped water and two peanut oil containers. VFA $$$R1$$$ mapping was performed in MS subjects to study the effect of different $$$B1$$$ mapping approaches on $$$R1$$$ quantification ($$$\rm{FA}=4/24^o,\rm{TR}=20\,\rm{ms}$$$). To exclude contributions from other potential mechanisms, modified imaging experiments (results not shown here) were also performed to control for other possible sources of image artifacts including vibrational motion (by decoupling the phantom from the table/coil through external support) and Eddy currents (by derating the spoiling gradients). Multi-echo AFI was used either in standard (the first echo processing) or in the proposed CSE regime (separating AFI signals $$$S_1/S_2$$$ into F/W followed by AFI on each channel). The fat fraction map from the separation was used to guide the backward combination of F/W AFI maps into a single map for demonstration purposes (FF>0.9 was considered to contain fat-only tissue, otherwise water). Additionally, we explored standard approach for improving AFI maps quality by spatial smoothing.Results
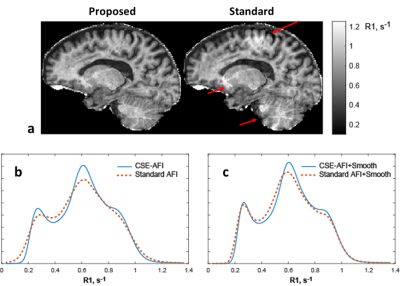
Figure 1 illustrates the proposed processing pipeline and correction of fat spoiling artifact in phantom. Results in Figure 2 further confirm that fat is the source of the $$$B1$$$ mapping artifacts observed in Fig. 1. Figure 3 compares the method’s performance in thighs and demonstrate removal of $$$B1$$$ mapping errors with the proposed CSE-AFI. Figure 4 demonstrates superior performance of CSE-AFI in brain in a variety of spoiling regimes. Figure 5 shows results of evaluation of different AFI methods for correction of excitation FA in VFA $$$R1$$$ mapping demonstrating that $$$B1$$$ with proposed CSE-AFI provides more complete correction of $$$R1$$$ value from flip angle variations.Discussion and Conclusions
This work identifies incomplete spoiling of fat signal as a major source of AFI $$$B1$$$ mapping errors. The standard approach to their correction necessitates infeasible requirements to gradient strength and/or scan time. Instead, we propose to use chemical-shift signature of the fat in conjunction with multi-echo AFI acquisition to eliminate the artifacts, thereby enabling $$$B1$$$ mapping with minimized errors even in standard spoiling regimen. While this method was demonstrated to improve significantly $$$B1$$$ mapping in locations with relatively limited amount of fat (e.g., in intracranial imaging), we anticipate the method to be especially useful in body imaging applications, where fat is more abundant.Acknowledgements
The work was supported by NIH (R01EB027087) and GE Healthcare.References
1. Yarnykh VL. Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn Reson Med 2007;57(1):192-200.
2. Yarnykh VL. Optimal radiofrequency and gradient spoiling for improved accuracy of T1 and B1 measurements using fast steady-state techniques. Magn Reson Med 2010;63(6):1610-1626.
3. Nehrke K. On the steady-state properties of actual flip angle imaging (AFI). Magn Reson Med 2009;61(1):84-92.
4. Steidle G, Eibofner F, Schick F. Quantitative diffusion imaging of adipose tissue in the human lower leg at 1.5 T. Magn Reson Med 2011;65(4):1118-1124.
5. Leupold J, Hennig J, Scheffler K. Moment and direction of the spoiler gradient for effective artifact suppression in RF-spoiled gradient echo imaging. Magn Reson Med 2008;60(1):119-127.
6. Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med 2008;60(5):1122-1134.
Figures