0662
A Correlational Study of Changes in Behaviour and MRI Measures in the CNS in the EAE Mouse Model of Multiple Sclerosis1University of Calgary, Calgary, AB, Canada, 2Department of Radiology, University of Calgary, Calgary, AB, Canada
Synopsis
Keywords: Multiple Sclerosis, Brain
Multiple sclerosis is an inflammatory disease of the central nervous system, with a posited hypoxia-inflammation cycle hypothesis. Hypoxia may increase disease severity in MS. We found R2* was increased (an MRI marker of hypoxia) in the cortex. We observed increased sickness and R2* in EAE mice compared to controls, but reduced CBF in both CFA-PTx and EAE. Inflammation is in both groups, while autoimmunity is only in EAE. Reduced CBF may relate to inflammation, whereas hypoxia may relate to autoimmune-mediated damage and increase disease severity.Introduction
Multiple sclerosis (MS) is a chronic and debilitating neurodegenerative disease of the central nervous system (CNS). MS is characterized by systemic inflammation contributing to local CNS inflammation1 through mediators triggering a proinflammatory state and excessive release of inflammatory cytokines.2 Hypoxia has been detected in the inflammation induced autoimmune mouse model of MS, the EAE.3 A hypoxia-inflammation cycle has been proposed, whereby inflammation promotes hypoxia and hypoxia may exacerbate inflammation, contributing to disease progression.2Non-invasive MRI markers to assess hypoxia and disease progression in MS are needed. Arterial spin labeling (ASL) is one such non-invasive marker which has been used to quantify cerebral blood flow (CBF); in the context of MS, reduced CBF has shown to precede cerebral anatomical changes following diagnosis.2
We used gradient echo MRI to quantify R2*, a marker of deoxyhemoglobin to assess hypoxia; R2* increases with increased deoxyhemoglobin (dHb). CBF was measured using ASL. We compared locomotor activity with ASL and R2* to evaluate if sickness relates to either MRI measure.
Methods
C57BL/6 female mice (n=30) were equally divided into three groups. On induction day, EAE mice were administered intraperitoneal injections of MOG35-55 peptide (50 µg/mL) emulsified in complete Freund’s adjuvant (CFA; 10 mg/ml of head inactivated mycobacterium tuberculosis emulsified in an antigen solution) and pertussis toxin (PTx; 300 ng/250 µL). CFA-PTx mice received the same injections, barring MOG administration. Two days post-induction, EAE & CFA-PTx mice received a PTx booster injection. Naive mice received no injections.To test for motor dysfunction, we used a 15-point scale. Loss of function in each limb merits a 3-point score and 2 points for the tail. A cumulative of 14 and 15 denote a paraplegic and dead mouse, respectively. We implemented an open field (OF) test as a behavioural measure to which the mice are initially habituated for a 5-minute interval on two occasions prior to induction. A baseline OF measurement took place prior to induction, a mid-study measurement 7-days post induction, and a third at peak sickness for EAE mice prior to sacrifice and subsequent imaging (day 17-21).
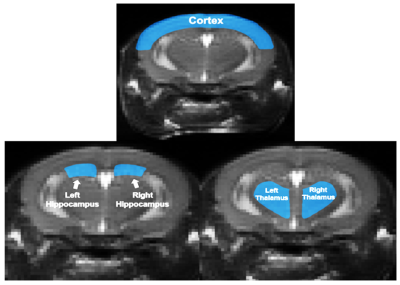
Groups were imaged using a 9.4T MRI Bruker Avance console with a Bruker Cryoprobe. Images were acquired with continuous ASL (cASL) (matrix size = 256 x 72, field of view = 25.6 mm x 25.6 mm, repetition time = 3000 ms, time to echo = 13.5 ms). A 3D multi-gradient echo sequence was used for R2*(matrix = 128x106x62, FOV = 19.2 x 15.9 x 9.3, TR = 100 ms, TE = 3.1 ms, echo spacing = 4 ms, pulse angle: 20º). dHB is positively correlated with R2*4. A one-way ANOVA and Bonferroni post-hoc statistical analysis was used to compare between groups.
Results
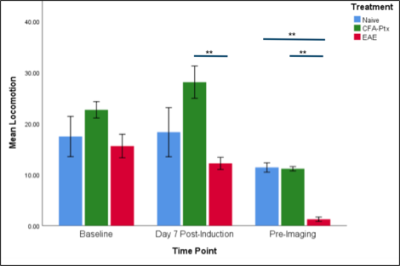
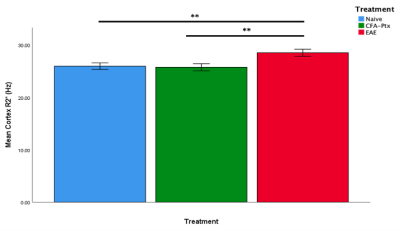
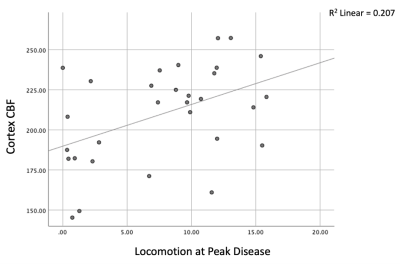
Sickness behaviour onset was observed in EAE mice starting on post-induction day 7, and was followed by a progressive increase in scoring; comparatively, CFA-PTx and Naive mice did not show an increase in behavioural scoring. Locomotion decreased in EAE compared to controls at mid-study (p = .002) and peak disease (p <.001). There was a significant decrease in CBF in the CFA-PTx (p = .012) and EAE (p = .040) mice compared to Naive. A significant decrease in CBF was also observed in the thalamus. Cortex R2* increased in EAE compared to both Naive (p = .047) and CFA-PTx (p = .023). A significant correlation was observed between sickness behaviour (distance traveled) and cortex CBF, r (29) = .455, p = .012 (Figure5).Discussion
We showed that EAE results in significant reductions in locomotion and CBF, as well as an increase in brain R2* using in-vivo 9.4T MRI and open-field behavioral tests. Deficits in locomotor activity may also be associated with loss of motor function and sickness which occurs in EAE. Moreover, we showed that reduced CBF occurs in both CFA-PTx and EAE mice – both of which possess inflammation, but not in the naïve (healthy mice) model. It is possible that inflammation results in cerebrovascular damage which can contribute to reduced vasoreactivity and hence reductions in CBF2. We only saw an increase in cortical R2* in the EAE model, but not the CFA-PTx. As an increase in R2* is consistent with an increase in deoxyhemoglobin, this is a possible marker for hypoxia. This change in R2* was not observed in CFA-PTx mice, possibly because CFA-PTx mice experience only systemic inflammation, which may not be sufficient for hypoxia development, while the EAE mice experience autoimmune-mediated CNS inflammation which may be severe enough to cause reductions in CBF as well as brain hypoxia.Conclusion
Increased R2* supports the theory that there is hypoxia in the brain of the EAE model of MS. This hypoxia relates to increased behavior deficits and reduced CBF, and so may relate to impairment and tissue damage in MS.Acknowledgements
JD received funding from Natural Sciences and Engineering Research Council(RGPIN-2015-06517), Canadian Foundation for Innovation, and Brain Canada. RM was funded by Alberta MS Network.References
Perry V. H. (2004). The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain, behavior, and immunity, 18(5), 407–413. https://doi.org/10.1016/j.bbi.2004.01.004
Yang, R., & Dunn, J. F. (2019). Multiple sclerosis disease progression: Contributions from a hypoxia-inflammation cycle. Multiple sclerosis (Houndmills, Basingstoke, England), 25(13), 1715–1718. https://doi.org/10.1177/1352458518791683
Johnson, T. W., Wu, Y., Nathoo, N., Rogers, J. A., Wee Yong, V., & Dunn, J. F. (2016). Gray Matter Hypoxia in the Brain of the Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis. PloS one, 11(12), e0167196. https://doi.org/10.1371/journal.pone.0167196
Figures