0659
Sex chromosomes and sex hormones differentially mitigate radiation-induced neuroanatomic deficits in Ccl2 knockout mice.1Translational Medicine, The Hospital for Sick Children, Toronto, ON, Canada, 2Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3The Hospital for Sick Children, Toronto, ON, Canada, 4Wellcome Centre for Integrative Neuroimaging, University of Oxford, Oxford, United Kingdom, 5Department of Paediatrics, The Hospital for Sick Children, Toronto, ON, Canada, 6Ontario Institute for Cancer Research, Toronto, ON, Canada
Synopsis
Keywords: Neuroinflammation, Preclinical, mouse, structural, radiation, Ccl2
Females tend to exhibit worse cognitive outcomes than males after pediatric cranial radiation therapy (CRT). Previous literature suggest that these sex differences may be driven by neuroinflammatory responses that involve CCL2. The objective of this study was to determine whether protection from Ccl2 knockout is mediated by sex hormones or sex chromosomes. We employ MRI on a CRT mouse model with mixed sex hormone and chromosome complements (i.e., four core genotypes model) to determine neuroanatomical changes. We found that both male chromosome and male sex hormones separately benefit regions of the brain in CCL2 deficient mice after CRT.Introduction
Pediatric cranial radiation therapy (CRT) results in neuroanatomical deficits detectable using MRI1. Females have a greater risk of severe cognitive impairment following CRT.2 One possible mechanism is a sex-dependent neuroinflammatory response.3,4 In a previous study, we decreased CCL2 expression, a chemokine that is responsible for recruiting immune cells to the brain. We showed that male mice deficient in CCL2 exhibited improved long-term outcomes after CRT-induced damage compared to females.5 To further elucidate the mechanisms driving these sex differences, we employ the four core genotypes mouse model in which the Y chromosome and its sex-determining region (Sry) are inherited separately, producing XY or XX chromosomes with or without Sry (gonadally and hormonally male or female, respectively).6 We use in vivo structural MRI to show that the presence of either XY or Sry differentially mitigates radiation-induced brain volume deficits in Ccl2-/- mice.Methods
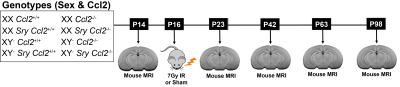
C57BL6/J mice with a homozygous knockout of Ccl2 or wildtype Ccl2 received whole-brain radiation (7Gy) or sham radiation (0Gy) at postnatal day (P)16. These mice were further divided into the four core genotypes (XY- SRY, XX SRY, XY-, and XX). In total, there are 16 groups (N=10–17 mice per group). Mice were scanned at P14, P23, P42, P63, and P98 (see Figure 1 for the experimental timeline). Manganese-enhanced in vivo images were acquired on a multi-channel 7T MRI scanner7 using a 3D T1-weighted gradient echo sequence with the following parameters: 75μm isotropic resolution, TR=26ms, TE=8.25ms, flip angle=26°, field-of-view=25×22×22mm, matrix size=334×294×294. Images were segmented into 183 structures using an automated registration pipeline.8,9 Structure-wise analysis was performed with a linear mixed-effects model. The fixed effects included age, gonadal sex, chromosomal sex, Ccl2 genotype, irradiation, and interactions for gonadal sex:Ccl2, chromosomal sex:Ccl2, gonadal sex:Ccl2:irradiation, and chromosomal sex:Ccl2:irradiation. Moreover, each term was modeled with an initial offset term and a slope linear in age. Biological variability was accounted for by including random intercepts for each mouse.Results
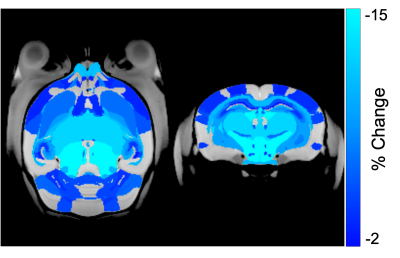
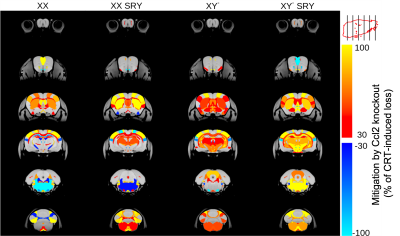
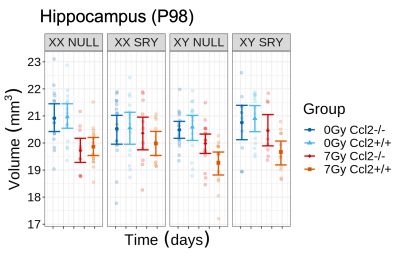
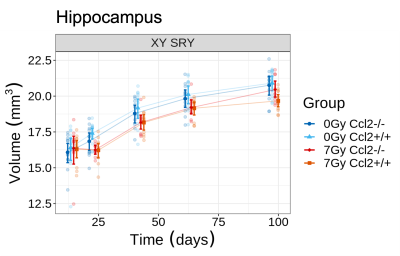
CRT induced widespread brain volume loss (Figure 2). Figure 3 shows the change in the radiation-induced deficit attributed to the four sex genotypes in Ccl2-/- mice at P98. The most prominent mitigation of radiation-induced volume loss occurred in mice with both XY- and Sry. XY and XX Sry Ccl2-/- mice exhibited partial recovery in several structures, such as the hippocampus (Figure 4), hypothalamus, thalamus, pons, midbrain, pituitary gland, and medulla. Ccl2-/- mice with an XY Sry genotype appeared to have the greatest overall brain volume recovery at P98, including the hippocampus (Figure 5).Discussion
Males are less susceptible to impairments in cognitive function compared to females after CRT.10–12 Sex differences arise early in CNS development with immune cells responsible for producing sexually dimorphic brain features.13,14 Furthermore, sex chromosomes and sex hormones independently mediate structural brain volumes throughout development.15 Our observations demonstrate spatial dependencies of sex chromosomes and sex hormones in the radiation-induced immune response. We found that the presence of either XY or Sry in Ccl2 knockout mice is beneficial to the brain after irradiation. These results provide clarification on the role and mechanisms of sex-induced differences in radiation response.Conclusion
Females tend to have poorer cognitive outcomes following cranial irradiation. To probe the mechanism of sex differences in radiation response, we showed that the presence of male sex hormones and male sex chromosomes can mitigate radiation-induced brain deficits in Ccl2 knockout mice. These results provide important insight as to why females may be more sensitive to radiation than males. This highlights the need for sex-specific strategies to minimize the risk of severe cognitive deficits.Acknowledgements
This work is supported in part by the Canadian Institute of Health Research, the Ontario Institute for Cancer Research, and a Restracomp award administered by the SickKids Research Training Centre.References
1. Nieman, B. J. et al. White and Gray Matter Abnormalities After Cranial Radiation in Children and Mice. International Journal of Radiation Oncology*Biology*Physics 93, 882–891 (2015).
2. Armstrong, G. T., Sklar, C. A., Hudson, M. M. & Robison, L. L. Long-Term Health Status Among Survivors of Childhood Cancer: Does Sex Matter? JCO 25, 4477–4489 (2007).
3. Hanamsagar, R. & Bilbo, S. D. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. The Journal of Steroid Biochemistry and Molecular Biology 160, 127–133 (2016).
4. Han, J., Fan, Y., Zhou, K., Blomgren, K. & Harris, R. A. Uncovering sex differences of rodent microglia. J Neuroinflammation 18, 74 (2021).
5. de Guzman, A. E. et al. Protection From Radiation-Induced Neuroanatomic Deficits by CCL2 Deficiency Is Dependent on Sex. International Journal of Radiation Oncology*Biology*Physics 113, 390–400 (2022).
6. Arnold, A. P. & Chen, X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Frontiers in Neuroendocrinology 30, 1–9 (2009).
7. Arbabi, A. et al. Multiple-mouse magnetic resonance imaging with cryogenic radiofrequency probes for evaluation of brain development. NeuroImage 252, 119008 (2022).
8. Friedel, M., van Eede, M. C., Pipitone, J., Chakravarty, M. M. & Lerch, J. P. Pydpiper: a flexible toolkit for constructing novel registration pipelines. Front. Neuroinform. 8, (2014).
9. Chakravarty, M. M. et al. Performing label-fusion-based segmentation using multiple automatically generated templates: MAGeT Brain: Label Fusion Segmentation Using Automatically Generated Templates. Hum. Brain Mapp. 34, 2635–2654 (2013).
10. Villasana, L., Acevedo, S., Poage, C. & Raber, J. Sex- and APOE Isoform-Dependent Effects of Radiation on Cognitive Function. Radiation Research 166, 883–891 (2006).
11. Roughton, K., Kalm, M. & Blomgren, K. Sex-dependent differences in behavior and hippocampal neurogenesis after irradiation to the young mouse brain: Gender differences after IR to the rodent brain. European Journal of Neuroscience 36, 2763–2772 (2012).
12. Christie, D., Leiper, A. D., Chessells, J. M. & Vargha-Khadem, F. Intellectual performance after presymptomatic cranial radiotherapy for leukaemia: effects of age and sex. Archives of Disease in Childhood 73, 136–140 (1995).
13. Lenz, K. M., Nugent, B. M., Haliyur, R. & McCarthy, M. M. Microglia Are Essential to Masculinization of Brain and Behavior. Journal of Neuroscience 33, 2761–2772 (2013).
14. Schwarz, J. M. & McCarthy, M. M. Cellular mechanisms of estradiol-mediated masculinization of the brain. The Journal of Steroid Biochemistry and Molecular Biology 109, 300–306 (2008).
15. Vousden, D. A. et al. Impact of X/Y genes and sex hormones on mouse neuroanatomy. NeuroImage 173, 551–563 (2018).
Figures