0653
Disrupted Associations between the Transcriptome Profile, Brain Structure and Function, and Cognitive Ability in Schizophrenia1Fourth Military Medical University, Xi'an, China, 2Xi’an People’s Hospital (Xi’an Hourth Hospital), Xi'an, China
Synopsis
Keywords: Psychiatric Disorders, Genetics, Schizophrenia
Cognitive dysfunction is considered to be one of the core symptom dimensions of schizophrenia. To deconstruct the underlying etiological behind cognitive deficits from a more integrated and multidimensional perspective, we established a trans-scale dataset at the level of the individual corresponding to each participant and control, containing blood-sample transcriptome profile, neuroimaging endophenotypes, and cognitive ability. Multivariate correlation analysis and mediation analysis demonstrated that the gene-brain-cognition associations were exsisted in healthy controls, whereas disrupted in patients with schizophrenia. These results provide a preliminary clue in comprehending the misaligned relationship between the microscale biological pathways and macroscopic phenotypes in schizophrenia.
Introduction
Cognitive impairments are one of the core symptoms of schizophrenia. However, how this impediment is associated with gene expression profile alterations and brain endophenotypes variations remains poorly understood.Methods
A trans-scale dataset containing whole-blood mRNA sequence data, brain magnetic resonance imaging data, and cognitive data was collected from 43 patients with schizophrenia and 60 healthy individuals. The count data of genes were obtained through extracted total mRNA data from whole blood samples1,2 and were used to calculate differentially expressed genes between patients and controls3. Based on the Wechsler Adult Intelligence Scale revised in China, the raw scores of six subtests including vocabulary, information, digit span, picture completion, block design, and digit symbol coding were administered. Raw scores were then converted to normalized scores in order to evaluated IQ. An independent cohort including 117 patients with schizophrenia and 125 healthy controls was applied to verify the primarily variational neuroimaging phenotypes. Based on the contribution of grey matter volume alterations to functional dysconnectivity4,5, we selected the brain region with the most significant altered grey matter volume in patients with schizophrenia compared with healthy controls as a region of interest for seed-based voxel-wise functional connectivity at whole-brain level based on a data-driven manner. Then, the effective connectivity was characterized to determine the information flow within brain regions which display dysfunction of networks in schizophrenia. Furthermore, to spatially match gene expression data with neuroimaging data, we localized differential genes to specific brain regions (referred to as Target genes) utilizing regional brain gene expression data in the Allen Human Brain Atlas (http://human.brain-map.org/). Finally, the relationships between the Target genes transcriptome Profile, neuroimaging phenotypes, and cognitive ability were measured separately in patients and controls based on the partial least squares analysis (https://github.com/danizoeller/myPLS) and the mediation analysis6.Results
Relative to healthy controls, patients with schizophrenia exhibited the most significant decreased grey matter volume in the right hippocampus/parahippocampal (HIP/PHG) grey, reduced strength of bidirectional excitatory effective connectivity between the right HIP/PHG and right superior temporal gyrus, and increased inhibitory connectivity within right HIP/PHG and left middle temporal gyrus (Figure 1). As for the relationship between brain phenotypes and cognition ability, the strength of the bidirectional effective connectivity between right HIP/PHG and right superior temporal exhibited positive correlation with digit symbol coding test score and negative correlations with the digit span test score as well as IQ in healthy controls, but demonstrated abnormal negative correlation with information test score in patients with schizophrenia (Figure 2). In healthy individuals, gene-brain-cognition association analysis established the relationships between several Target gene expression levels and brain functional parameters & cognitive ability (r = 0.4797, P = 0.0002) on the one hand, and mediation analysis further disclosed the mediating effect (indirect effect = 0.0242, 95% CI from the bootstrap test = [0.0005 to 0.0524]) of brain phenotypes composite scores, which is principally contributed by the bidirectional connections between the right HIP/PHG and superior temporal gyrus, in the correlation between gene expression and cognitive composite scores on the other hand (Figure 3). Crucially, in compared with healthy controls, the normal trans-scale associations described above were extensively disrupted in patients with schizophrenia.Discussion
The worse information test was connoted the dysfunction of verbal comprehension7. Verbal comprehension is primarily applied to measure the capacity for the comprehension of specific task and recognization of abstract concepts, which relies on the information input and integration process8,9. Remarkably, effective information transmission and encoding processes are largely dependent on the balance and stability of trans-synaptic signalling and synapse configuration which are precisely regulated by genetic factors and play critical roles in the pathology of schizophrenia10-13. Trans-scale associations between blood expression levels of several target genes, including WDR1, NLGN2, and ADAM8, bidirectional effective connectivity strength between the right HIP/PHG and right superior temporal gyrus, as well as cognitive test score were demonstrated high correlation coefficients in healthy individuals but disordered in schizophrenia. Notably, these genes play a significant role in regulating synaptic plasticity, maturation, and function, with further implications for cognitive ability14-18. To some extent, these studies further suggest that distorted gene expression in schizophrenia could exert impact on the function of synapses, thus might lead to activation and coordination impairment between brain regions, which ultimately manifests as information processing dysfunction. More importantly, based on the results of the mediation analysis, the association between gene composite score and cognition composite score was exclusively mediated by brain phenotypes composite score, which was present in healthy individuals, whereas absent in patients with schizophrenia. Our findings have implications for understanding the triple relationship among genetic factors, brain function, and cognitive ability related to healthy individuals, and meanwhile shed new light on the neuropathological model manifesting the disorganized relationship of gene-brain-cognition in patients with schizophrenia.Conclusion
In conclusion, this study linked HIP/PHG structure and function as well as cognitive ability to the blood transcription levels of HIP/PHG related genes at individual level without extracting brain tissue, providing a new perspective for understanding the misaligned gene-brain-cognition biological pathway in schizophrenia. Our findings have initially identified a potential neuropathological model probably associated with cognitive dysfunction in schizophrenia, including both genetics and brain function mechanisms.Acknowledgements
We thank Prof. Hua-Ning Wang for supporting this study.
References
1. Chen S, Zhou Y, Chen Y, et al. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34(17):i884-i890.
2. Kim D, Langmead B, Salzberg S L. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357-360.
3. Love M I, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
4. Koch K, Rus O G, Reeß T J, et al. Functional connectivity and grey matter volume of the striatum in schizophrenia. Br J Psychiatry. 2014;205(3):204-213.
5. Luo N, Sui J, Chen J, et al. A Schizophrenia-Related Genetic-Brain-Cognition Pathway Revealed in a Large Chinese Population. EBioMedicine. 2018;37:471-482.
6. Preacher K J, Hayes A F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879-891.
7. Yao S, Chen H, Jiang L, et al. Replication of factor structure of Wechsler Adult Intelligence Scale-III Chinese version in Chinese mainland non-clinical and schizophrenia samples. Psychiatry and clinical neurosciences. 2007;61(4):379-384.
8. Fujino H, Sumiyoshi C, Sumiyoshi T, et al. Performance on the Wechsler Adult Intelligence Scale-III in Japanese patients with schizophrenia. Psychiatry and clinical neurosciences. 2014;68(7):534-541.
9. Maseda A, Lodeiro-Fernández L, Lorenzo-López L, et al. Verbal fluency, naming and verbal comprehension: three aspects of language as predictors of cognitive impairment. Aging Ment Health. 2014;18(8):1037-1045.
10. Matosin N, Fernandez-Enright F, Lum J S, et al. Molecular evidence of synaptic pathology in the CA1 region in schizophrenia. NPJ Schizophr. 2016;2:16022.
11. Volk L, Chiu S-L, Sharma K, et al. Glutamate synapses in human cognitive disorders. Annual Review of Neuroscience. 2015;38:127-149.
12. Owen M J, Sawa A, Mortensen P B. Schizophrenia. Lancet. 2016;388(10039):86-97.
13. Forsyth J K, Lewis D A. Mapping the Consequences of Impaired Synaptic Plasticity in Schizophrenia through Development: An Integrative Model for Diverse Clinical Features. Trends In Cognitive Sciences. 2017;21(10):760-778.
14. Wang J, Kou X-L, Chen C, et al. Hippocampal Wdr1 Deficit Impairs Learning and Memory by Perturbing F-actin Depolymerization in Mice. Cereb Cortex. 2019;29(10):4194-4207.
15. Jickling G C, Ander B P, Zhan X, et al. Progression of cerebral white matter hyperintensities is related to leucocyte gene expression. Brain. 2022;145(9):3179-3186.
16. Katzman A, Alberini C M. NLGN1 and NLGN2 in the prefrontal cortex: their role in memory consolidation and strengthening. Curr Opin Neurobiol. 2018;48:122-130.
17. Sun C, Cheng M-C, Qin R, et al. Identification and functional characterization of rare mutations of the neuroligin-2 gene (NLGN2) associated with schizophrenia. Hum Mol Genet. 2011;20(15):3042-3051.
18. Ali H, Marth L, Krueger-Burg D. Neuroligin-2 as a central organizer of inhibitory synapses in health and disease. Sci Signal. 2020;13(663).
Figures
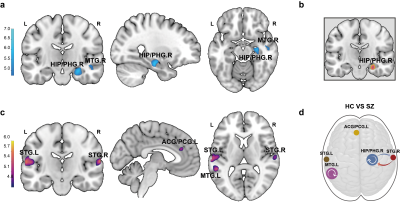
Figure 1. (a) Brain areas with decreased grey matter volume in patients compared with healthy controls (FWE correction; adjusted P < 0.05). (b) The cluster was selected for the voxel-wise functional connectivity analysis. (c) Brain regions with decreased functional connectivity in patients compared with healthy controls (FWE correction; adjusted P < 0.05). (d) Decreased excitatory effective connectivity (blue and orange) and increased inhibitory connectivity (white) in patients with schizophrenia compared with healthy controls.
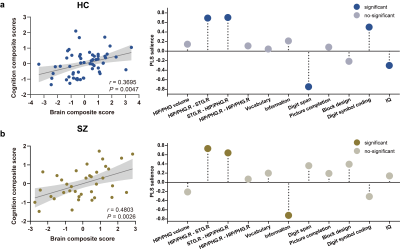
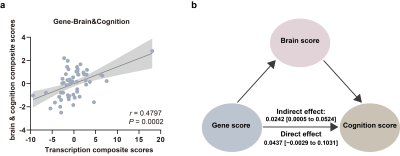
Figure 3. (a): Target genes transcription composite score were significantly correlated with brain & cognition composite score in healthy controls. (b): The result from the mediation effect of Target gene expression on cognition through brain function in healthy controls.