0648
Metabolic changes in hippocampus in preclinical model due to chronic noise exposure: An in-vivo 1H Magnetic Resonance Spectroscopy study.
Nisha Chauhan1, S Senthil Kumaran1, and Himanshu Singh1
1Department of Nuclear Magnetic Resonance, All India Institute of Medical Sciences, New Delhi, India
1Department of Nuclear Magnetic Resonance, All India Institute of Medical Sciences, New Delhi, India
Synopsis
Keywords: Psychiatric Disorders, Brain, Stress, Depression, Pre clinical, MRS
Occupational and environmental noise contributes to major public health issues. The chronic high level noise exposure may cause stress and depression and also alterations in Neurometabolites. In-vivo 1H MRS and Open-Field Test are carried out to elucidate the changes in brain metabolite and its effect on behavior due to chronic noise exposure in a preclinical model. A significant increase in Glutamate and N- Acetylaspartate metabolite was observed in hippocampus which is an important target to study the effects of stress. Reduced total movement time, movement distance and mean velocity were observed in the noise exposed group.Introduction
Environmental noise exposure is a major global public health problem, with consequences in physical health, contentment, and longevity1. The effect of noise depends on sound intensity, frequency and duration of exposure. Noise stress has been shown to alter neurotransmitter levels in the brain2 and reduce dendrite growth that implicates impaired memory and cognition in rats3. As humans cannot be exposed to long-term high levels of noise for experimental purpose, this study was conducted in rats. The present aim of the study was to better understand the impact of chronic noise exposure on brain metabolites and behavior in an experimental model.Methods
30 Wistar rats (230-330gm, male) were divided into 2 groups, Sham group (n=15) kept in normal condition and Noise-exposure group (n=15) exposed to >100db white noise up to 3 hrs daily for 21-23 days in an audio booth. The behavioural changes were assessed using Open field test (Coulbourn, M/s Harvard Apparatus Inc., USA, video recording system of Truscan, M/s Harvard Apparatus Inc., USA) and after that MR spectra were acquired using Small Animal 7T MR scanner (Biospec, M/s Bruker Biospin GmBH, Germany), on Day 0 and Day 21-23 after exposure.Open Field Test
In open field test, rats were allowed to move around freely in a quiet environment. The apparatus was a cubic box with 40x40x40cm dimension, with glass walls, black floor and photo-sensor beams at the bottom for tracking activity. After exposure of rats to noise (Figure 1(a)), each rat was gently placed in the apparatus (Figure 1(b)) and its behavior was video-recorded for 5 min. Open field test was scored by using TruScan software (M/s Harvard Apparatus Inc., USA). Indicators of exploratory behavior such as Total movement time, total movement distance and mean velocity were estimated.
Magnetic Resonance Spectroscopy
Rats were anesthetized with isoflurane/O2 mixture and then placed inside a 40 mm volumetric head coil in 7 Tesla MRI machine (70/20 US Biospec, M/s Bruker Biospin GmBH). Single voxel MRS spectra in the right hippocampus region (voxel size: 2x2x4mm) was planned on T1 weighted image of rat brain as shown in Figure 2(a) and acquired with TR/TE:2500/16.6 ms, 256 averages, 2048 points, VAPOUR water suppression.
Spectral Analysis: In vivo spectra were analysed in reference to a simulated basis set provided by the LC Model (Figure 2(b)). Water and eddy current corrections were carried out with unsuppressed spectrum containing the water signal (with 8 averages), acquired from the same voxel. After frequency and phase correction of individual FID of MRS signal, quantitative metabolite analysis was done in the chemical shift range of 0.0–4.0 ppm and the Cramer-Rao lower bounds (CRLB) were used to eliminate statistically unreliable values.
Statistical Analysis: Statistical analysis was done using SPSS (version 22). Independent Mann-Whitney U test was used to analyse the absolute difference in metabolite levels and open field test parameters such as move time, velocity and distance covered, between sham and noise exposed group. A value of p<0.05 was considered to indicate a significant difference amongst groups.
Results
The concentration of glutamate (U = 69, p = .026) and N-Acetylaspartate (U = 68.5, p = .023) in right hippocampus were significantly higher in noise exposed group as compared to the sham group (Figure 3(a), Table 1). Analysis of the open field test data revealed significantly reduced movement activity, Move time (U = 40.5, p = .004), Distance (U = 36, p = .002), and Velocity (U = 34, p = .001) of noise exposed rats in comparison to sham group (Figure 3(b), Table 1).Discussion
The hippocampus is an important target to study the effect of stress3, as stress-induced alterations in hippocampal neurons are reported to underlie cognitive and memory deficits4. Metabolic analysis of hippocampal region in Chronic mild stress (CMS) rat model has revealed an increase in the levels of N-acetylaspartate and glutamate5. The present study suggests that stress induced due to noise exposure cause an increase in N-acetylaspartate and glutamate levels in the hippocampus region in concurrence with a previous study5. Noise exposure also affected behavior of rats by reducing their total movement time, distance covered and mean velocity6.Conclusion
This study reveals an increase of brain metabolites such as N-acetylaspartate and Glutamate, which are markers of neuronal density, neurogenesis, or neuronal differentiation, in the hippocampus region of noise exposed rats and may be attributed to stress and anxiety.Acknowledgements
The funding from Life Sciences Research Board (LSRB), Ministry of Defence, Government of India is duly acknowledged (vide grant No. LSRB-295/PEE&BS/2017)References
- Van Dijk et.al. (1987). Non-auditory effects of noise in industry - VII. Evaluation, conclusions and recommendations. International Archives of Occupational and Environmental Health, 59(2).
- Ahmad, A., Rasheed, N., Banu, N., & Palit, G. (2010). Alterations in monoamine levels and oxidative systems in frontal cortex, striatum, and hippocampus of the rat brain during chronic unpredictable stress. Stress (Amsterdam, Netherlands), 13(4), 355–364.
- Manikandan, S., Padma, M. K., Srikumar, R., Jeya Parthasarathy, N., Muthuvel, A., & Sheela Devi, R. (2006). Effects of chronic noise stress on spatial memory of rats in relation to neuronal dendritic alteration and free radical-imbalance in hippocampus and medial prefrontal cortex. Neuroscience letters, 399(1-2), 17–22.
- Kraus, K. S., Mitra, S., Jimenez, Z., Hinduja, S., Ding, D., Jiang, H., Gray, L., Lobarinas, E., Sun, W., & Salvi, R. J. (2010). Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience, 167(4), 1216–1226.
- Akimoto, H., Oshima, S., Sugiyama, T., Negishi, A., Nemoto, T., & Kobayashi, D. (2019). Changes in brain metabolites related to stress resilience: Metabolomic analysis of the hippocampus in a rat model of depression. Behavioural brain research, 359, 342–352.
- Katz, R. J., Roth, K. A., & Carroll, B. J. (1981). Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neuroscience and biobehavioral reviews, 5(2), 247–251.
Figures

Figure 1(a): Noise Exposure of rats in their cages for 3hr daily; 1(b) Open Field Test done in Coulbourn Apparatus for locomotor activity of rat.
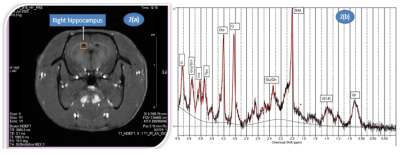
Figure 2(a): 1H MRS voxel placement on hippocampus region on T1 brain image of rat, 2(b) 1H MRS representative spectrum from the rat hippocampus analyzed using LC Model.
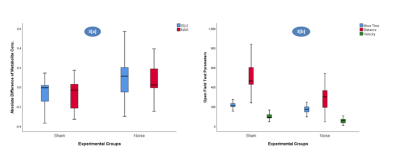
Figure 3(a): MRS metabolite levels of Glutamate and NAA in sham and experimental groups, 3(b) Open field test parameters - move time, distance covered and mean velocity in sham and experimental groups.
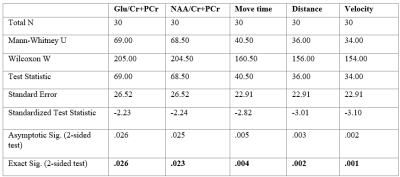
Table 1. Independent-Samples Mann-Whitney U Test across sham and experimental group for the measured parameters.
DOI: https://doi.org/10.58530/2023/0648