0647
Glymphatic system dysfunction in Primary Insomnia evidenced by diffusion tensor imaging with the perivascular space (DTI-ALPS)
Yu Jin1, Xiaoyong Zhang2, Xin Ding3, and Guangwen Chen1
1Department of Radiology, Chengdu Second Peolpe's Hospital, Chengdu, China, 2Clinical Science, Philips Healthcare, Chengdu, China, 3Department of Neurology, Chengdu Second Peolpe's Hospital, Chengdu, China
1Department of Radiology, Chengdu Second Peolpe's Hospital, Chengdu, China, 2Clinical Science, Philips Healthcare, Chengdu, China, 3Department of Neurology, Chengdu Second Peolpe's Hospital, Chengdu, China
Synopsis
Keywords: Psychiatric Disorders, Microstructure
Sleep has been hypothesized to assist waste clearance from the brain. We aimed to determine whether primary insomnia is associated with glymphatic system dysfunction by using diffusion tensor imaging (DTI) with the perivascular space (DTI-ALPS), a potential marker of impaired brain waste clearance. In this study, we found that the DTI-ALPS in patients with primary insomnia was significantly lower than in healthy controls and the DTI-ALPS index was significantly negatively correlated with neuropsychological performance score. The results suggests that DTI-ALPS may be a useful imaging tool for studying glymphatic system function with primary insomnia.Background
Primary insomnia is characterized by difficulties in falling asleep, maintaining sleep, and early morning awakening. Moreover, it is coupled with daytime consequences such as fatigue, attention deficits, mood instability and many serious disease [1]. The glymphatic system is a highly organized fluid transport pathway that clears cerebral protein waste products [2]. Several evidence shows that glymphatic fluid transport is robustly enabled by non-rapid eye movement (NREM) sleep and is suppressed during wakefulness. Glymphatic system dysfunction has been recently implicated in a variety of sleep diseases, such as isolated REM sleep behavior disorder [3], and there were also works demonstrated glymphatic system dysfunction in patients with obstructive sleep apnea by using diffusion tensor imaging (DTI) with the perivascular space (DTI-ALPS) [4]. This study aimed to evaluate the glymphatic system function in patients with primary insomnia compared to healthy controls using DTI-ALPS method. Our hypothesis is that patients with primary insomnia may have glymphatic system dysfunction, which is correlated with sleep, and neuropsychological performance score.Methods
This study was approved by the institutional ethics committee. We prospectively enrolled 16 patients with primary insomnia and 16 healthy controls. All participants underwent DTI magnetic resonance imaging (MRI) on a same 3T MRI scanner using 24-channel standard head coil (Ingenia DNA, Philips Medical Systems, Netherlands). DTI was conducted using spin-echo single-shot echo-planar pulse sequences with main parameters as follows: TR/TE = 4472/90 ms, flip angle = 90° , field of view = 256 mm ×256 mm, matrix = 240×240, reconstructed voxel size = 2×2×2 mm3, 64 axial slices with no gap, and acquisition time = 5 minutes. The diffusion sensitive gradients were applied along 64 noncollinear directions with 𝑏 value = 1000 s/mm 2 to obtain the weighted images and one unweighted B0 image with 𝑏 value = 0 s/mm2. The DTI-ALPS index was calculated from the images (Figure 1), and the differences in the DTI-ALPS index between patients with primary insomnia and healthy controls were finally evaluated. In addition, we conducted a correlation analysis between the DTI-ALPS index and sleep, neuropsychological performance score (including Pittsburgh Sleep Equality Index (PSQI), Insomia Severity Index (ISI), Epworth Sleep Scale (ESS)).Results
The DTI-ALPS index was significantly different between the groups. The DTI-ALPS in patients with primary insomnia was significantly lower than in healthy controls (t=4.24, p=0.00, Figure 2). Furthermore, the DTI-ALPS index was significantly negatively correlated with the PSQI score (r=-0.62, p=0.01, Figure 3).Conclusions
We successfully demonstrated glymphatic system dysfunction in patients with primary insomnia. In addition, glymphatic system dysfunction is well correlated with PSQI score. Thus, these findings can explain the effects of primary insomnia on increased risk of developing dementia and highlight the importance of primary insomnia treatment.Acknowledgements
This work was supported by the Chengdu Municipal Health Commission (no.2022054).References
1.Chung KF, Yeung WF, Ho FY, et al. Cross-cultural and comparative epidemiology of insomnia: the Diagnostic and statistical manual (DSM), International classification of diseases (ICD) and international classification of sleep disorders (ICSD). Sleep Med 2015;16(4):477e82.
2. Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol 2018;17(11):1016e24.
3. Lee DA, Lee HJ, Park KM. Glymphatic dysfunction in isolated REM sleep behavior disorder. Acta Neurol Scand. 2022 Apr;145(4):464-470.
4. Lee HJ, Lee DA, Shin KJ, et al. Glymphatic system dysfunction in obstructive sleep apnea evidenced by DTI-ALPS. Sleep Med. 2022 Jan; 89:176-181.
Figures
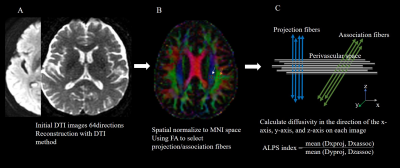
Figure1.Analysis process
from initial DTI data to calculate ALPS. A.DTI preprocessing(A). ROI placement
for calculation of the DTI-ALPS index. ROIs of 5x5mm2 were placed in
the projection (white arrow) and association areas (orange arrow) (B). indicates
the relationship between the direction of the perivascular space (gray lines) and
the directions of the fiber(C).
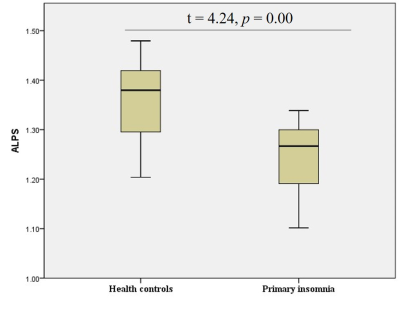
Figure2.Differences
between the glymphatic system functions of patients with primary insomnia and
healthy controls. The figure shows that the DTI-ALPS index of patients with primary
insomnia is significantly lower than that of healthy controls, suggesting that
patients with primary insomnia have glymphatic system dysfunction.
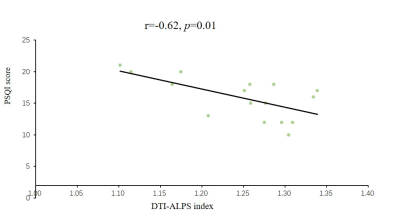
Figure
3. Correlation analysis between the DTI-ALPS index and PSQI score.
DOI: https://doi.org/10.58530/2023/0647