0645
ACC Connectivity Changes during ALIC Deep Brain Stimulation for Obsessive Compulsive Disorder
Sushil Bohara1, Natalya Slepneva2, Tenzin Norbu2, Moses Lee2, and Melanie A. Morrison3
1University of California, Berkeley, Berkeley, CA, United States, 2Department of Psychiatry & Behavioral Sciences, University of California, San Fransciso, San Francisco, CA, United States, 3Radiology & Biomedical Imaging, University of California, San Fransciso, San Francisco, CA, United States
1University of California, Berkeley, Berkeley, CA, United States, 2Department of Psychiatry & Behavioral Sciences, University of California, San Fransciso, San Francisco, CA, United States, 3Radiology & Biomedical Imaging, University of California, San Fransciso, San Francisco, CA, United States
Synopsis
Keywords: Brain Connectivity, Psychiatric Disorders
Chronic deep brain stimulation (DBS) of the anterior limb of the internal capsule (ALIC) is an emergent therapy for severe cases of obsessive-compulsive disorder (OCD), however patient response is variable with only 50-60% of patients classified as responders. Toward optimizing therapy for the individual patient and improving overall treatment efficacy, here we used fMRI during ALIC stimulation to evaluate changes in OCD network functional connectivity. In 5 patients, we found that DBS, when in a therapeutic configuration for the patient, may be reducing abnormal hyperconnectivity to improve symptoms, while nontherapeutic configurations may be disrupting fronto-posterior connections causing unwanted symptoms.Introduction
Deep brain stimulation (DBS), a surgical neuromodulation technique, has emerged as an adjunct therapy to treat patients with severe treatment-refractory obsessive-compulsive disorder (OCD).1 The DBS implant is comprised of a programmable neurostimulator placed under the skin of the chest that delivers continuous electrical impulses to a chosen brain target via unilateral or bilateral electrodes (Fig.1A). Following implantation, patients undergo months of trial-and-error programming to optimize DBS settings for maximum therapeutic benefit and minimum side-effects. For treatment of OCD, DBS is commonly targeted to the anterior limb of the internal capsule (ALIC), a white matter bundle that is structurally and functionally connected to key brain regions implicated in OCD such as the anterior cingulate cortex (ACC).2,3,4 Currently, only 50-60% of patients are responders to ALIC-DBS.5 This limited efficacy is in part due to the variability in patient symptoms and anatomy, but also a lack of biomarkers linking the functional brain response to DBS with symptom improvement which could guide therapy optimization for the individual patient. To address this need, we aimed to evaluate fMRI connectivity changes during ALIC-DBS using a simultaneous imaging and stimulation paradigm that is being increasingly utilized for DBS mechanism discovery and therapy optimization.Methods
Five patients (age >21 years) with treatment-refractory OCD receiving DBS as part of their standard clinical care consented to multiple 3T fMRI scans with simultaneous stimulation over one or more visits. All patients had 3T MR-compatible DBS systems (Medtronic Percept PC) with bilateral leads (Medtronic 3387 or 3991) in the ALIC and were scanned for a maximum of 30 minutes per visit. Imaging was performed with stimulation continuously cycling ON and OFF for 1-minute intervals, for a total of 6 minutes; this was repeated for different therapeutic and/or non-therapeutic bipolar electrode contact configurations, corresponding to spatially different volumes of brain tissue being targeted by DBS (Fig.1B-C). The gradient-echo fMRI data were acquired on a 3T GE scanner in low-SAR mode with a 32-channel head coil, TR/TE=2s/30ms, voxel size=3.75x3.75x4cm, flip angle=86, and FOV=24cm. Following acquisition, the data were preprocessed in the CONN toolbox, and connectivity maps seeded in the ACC were generated for the DBS OFF and ON conditions.6 The ACC was chosen given its propensity to be abnormally hyperconnected in patients with OCD.7 The final seed maps were thereafter used to evaluate DBS OFF versus ON effects on connectivity: 1) across all patients (N=5) and electrode configurations (N=26), 2) for electrode configurations classified as therapeutic (N=9) versus non-therapeutic contacts (N=17) based on their acute effects on symptoms and proximity to patients’ most clinically effective monopolar contact, and 3) for patients classified overall as responders (N=3) versus non-responders (N=2) to DBS based on clinical assessment of overall symptom improvement from baseline. For all maps, a voxel threshold of puncorrected<0.01 was used followed by a cluster size threshold of 20 voxels.Results
When contrasting DBS ON and OFF conditions across all patients and configurations, we observed a reduction in ACC connectivity with the bilateral precuneus (preC), L frontal pole, R fusiform gyrus, and L precentral gyrus (Fig.2). There were also notable increases in ACC connectivity with the L postcentral gyrus and L superior temporal gyrus. Upon comparing DBS ON and OFF conditions for electrode configurations that were therapeutic versus nontherapeutic, we found that (i) therapeutic stimulation was associated with a more prominent reduction in ACC connectivity with the bilateral middle frontal gyri (MFG), and (ii) nontherapeutic stimulation resulted in a larger reduction in ACC-preC connectivity (Fig.3). Further separation of the data based on whether patients were classified overall as responders or nonresponders to DBS showed subtle visible differences in the variability of ACC connectivity across DBS ON/OFF conditions (Fig.4). Specifically, responders appeared to have fewer larger fluctuations in connectivity along with more negative correlations between ACC and MFG fMRI signals for nontherapeutic configurations.Discussion
In this study, we identified several brain areas within the OCD network that are modulated by DBS. While some of these areas are known to be structurally connected to the ALIC through its frontal, sensorimotor, and cerebellar projections terminating in the frontal lobe, sensorimotor cortices, and cerebellum,8 connectivity changes in other areas may be driven by polysynaptic connections. While the therapeutic DBS mechanism for OCD is still unclear, these initial results suggest that ACC-MFG connectivity reduction by DBS may play a role in yielding therapeutic effects given that both areas are hyperactive in OCD and structurally connected. Interestingly, the preC has been associated with cognitive biases in OCD related to the thought of negative events occurring9 and here we showed greater disruption of ACC-preC connectivity when DBS was turned on during fMRI to nontherapeutic configurations that also caused anxiety and high energy. To further our understanding of these results, ongoing work is evaluating the impact of precise electrode location as it relates to the volume of tissue activated by DBS, since two of the patients had longer electrode models with more space between each contact, spanning more of the ALIC.Conclusion
Our results suggest that DBS, when in a therapeutic configuration for the patient, may be reducing abnormal hyperconnectivity to improve symptoms, while nontherapeutic configurations may be disrupting fronto-posterior connections causing unwanted symptoms.Acknowledgements
The authors would like to acknowledge the support of the MRI staff at the Surbeck Laboratory for Advanced Imaging.References
- Li, N. et al. A unified connectomic target for deep brain stimulation in obsessive-compulsive disorder. Nat. Commun. (2020), 11: p 3364.
- Dougherty, D. D. et al. Neuroscientifically Informed Formulation and Treatment Planning for Patients With Obsessive-Compulsive Disorder. JAMA Psychiatry. (2018), 75: p 1081.
- Baldermann, J. C. et al. Connectomic Deep Brain Stimulation for Obsessive-Compulsive Disorder. Biol. Psychiatry (2021)
- Nuttin B. et al. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. (1999), 354(9189): p 1526.
- Denys D, Graat I, Mocking R, et al. Efficacy of Deep Brain Stimulation of the Ventral Anterior Limb of the Internal Capsule for Refractory Obsessive-Compulsive Disorder: A Clinical Cohort of 70 Patients. AJP. (2020), 177(3): p 265-271.
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated BrainBrain Networks. Brain Connect. (2012), 2: p 125–141.
- Saxena, S., Brody, A., Schwartz, J., Baxter, L. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. British Journal of Psychiatry. (1998), 173(S35): p 26-37.
- Schüller T, Kohl S, Dembek T, et al. Internal capsule/nucleus accumbens deep brain stimulation increases impulse. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. Published online 2022.
- Jones R, Bhattacharya J. A role for the precuneus in thought-action fusion: evidence from participants with significant obsessive-compulsive symptoms. Neuroimage Clin.(2013), 28(4): p 112-21.
Figures
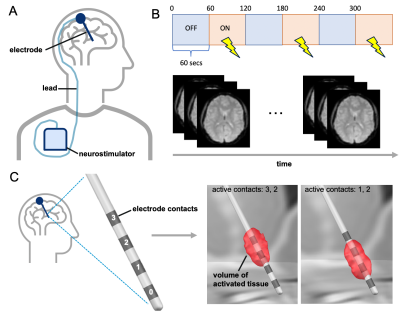
Figure 1: Patients implanted with a DBS device (A) underwent simultaneous fMRI and cycling stimulation (B) repeatedly for different DBS electrode configurations (C) that were therapeutic and/or non-therapeutic.
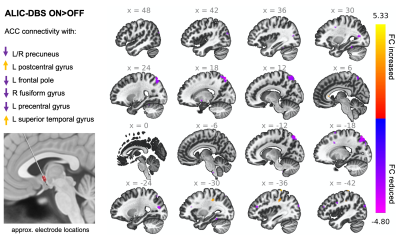
Figure 2: Stimulation of the ALIC in patients with OCD caused changes in ACC connectivity with multiple brain areas. Note: The voxel threshold was puncorrected<0.01 with an additional cluster size threshold for 20.
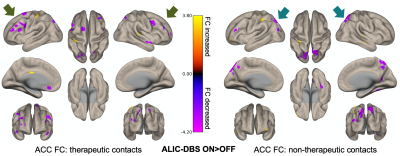
Figure 3: The effects of DBS were distinct for fMRI scans acquired during therapeutic versus nontherapeutic stimulation. ACC connectivity to frontal areas showed greater reductions with therapeutic stimulation, whereas its connectivity to posterior areas was more affected during nontherapeutic stimulation. Note: The voxel threshold was puncorrected<0.01 with an additional cluster size threshold for 20.
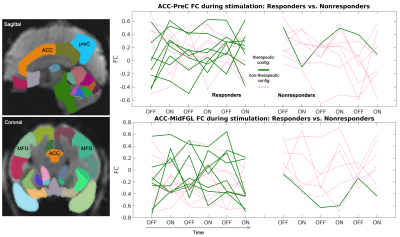
Figure 4: Qualitatively, there were subtle differences in ACC-MFG and ACC-preC connectivity across responder (N=3) and nonresponder (N=2) patients, who were scanned at different therapeutic (green) and nontherapeutic (pink) DBS configurations. Specifically, responders had fewer large fluctuations in ACC-MFG connectivity during nontherapeutic stimulation along with more negative correlations. Note: FC= functional connectivity. Each line corresponds to a different fMRI scan with a unique electrode configuration stimulating the brain. Patients are not differentiated.
DOI: https://doi.org/10.58530/2023/0645