0620
Whole brain Layer-fMRI on the NexGen 7T scanner with high performance gradients and 64-channel receiver array.1Helen Wills Neuroscience Institute, University of California, Berkeley, CA, United States, 2Advanced MRI Technologies, Sebastopol, CA, United States, 3Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands, 4Siemens Medical Solutions USA, Inc., Malvern, PA, United States, 5MR CoilTech Limited, Glasgow, United Kingdom, 6Imaging Centre of Excellence, University of Glasgow, Glasgow, United Kingdom
Synopsis
Keywords: fMRI, Data Acquisition
Laminar-resolved fMRI has the potential to capture directional information flow within and between cortical areas to inform network neuroscience. However, common layer-fMRI imaging protocols are constrained by:
- limited slab coverage
- commonly used 0.8mm resolution barely resolves individual layer groups.
In this abstract, we use the NexGen 7T scanner to develop a whole-brain functional imaging protocol at 0.6mm resolutions. The aim was to identify and mitigate challenges in protocol optimization of 3D-EPI VASO:
- maintaining CBV-weighting with long acquisition windows via segmentation across IR,
- residual EPI ghosts
- residual fat ghosts
We show whole-brain 0.64mm CBV-based connectivity maps covering the entire neocortex.
Introduction
Layer-fMRI studies have used several different imaging sequences (GE-EPI, GRASE, VASO) and are typically limited to partial brain coverage, either using zoomed Inner-volume techniques1,2, 3D slab volumes3 or a restricted number of slices in multi-slice 2D image acquisitions4. Generally there is a trade-off between brain coverage, TR and spatial resolution, which has made whole brain layer-fMRI very challenging to achieve given the requirement for sub-millimeter spatial resolution. Here we perform whole brain layer-fMRI taking advantage of the higher performance hardware on the Next Generation 7T scanner, which was developed specifically to achieve higher resolution in fMRI.Methods
MRI Scanner: Higher resolution is encoded without lengthening the echo spacing and echo train length using the scanner’s unique high performance gradient system, Impulse gradient (Siemens) with slew rate 900 T/m/s and capable of a maximum gradient 200 mT/m. The scanner also has a 128 channel receiver system and 16 channel transmitter, however these experiments used a 64 ch Rx, 8 ch Tx coil (MR CoilTec)5 which was built to fit into the 44cm diameter head only gradient coil.fMRI protocol: Movie-watching tfMRI was collected with the subject viewing video clips from the 7T HCP project . Each run lasted for 10 minutes and was repeated 5 times in Session 1 and 4 times in Session 2.
Acquisition Sequence: The imaging sequence was a VASO 3D segmented EPI sequence6,7,8 using 3D GRAPPA acceleration of 3 x 2 and multi-shot 2 segmentation on the in-plane axis. Parameters: 0.64 mm isotropic resolution, Volume TR = 4.2s TE= 20ms, echo spacing=0.69ms, BW= 1592 Hz, FOV= 200 x 200 mm, matrix size 314 x 314, 180 slices. The phase correction approach of Dual-polarity EPI was employed by alternating the polarity of the EPI switched read gradient waveform on alternate TRs9,10. In order to fulfill the VASO blood nulling condition despite T1-relaxation along the 3D EPI readout, four inversion pulses were used for each k-space volume. Sequence parameters were tested across 4 sessions with two participants. The sequence parameter choices used in the final protocol are based on exploratory pilot experiments depicted in Figs. 2 & 3.
Connectivity analysis: Time series were extracted for the common ICA networks11, they were orthogonalised and used as regressors for activation maps.
Reconstruction was done in SIEMENS’ IcePat in a temporal sliding window approach. Consecutive TRs of inverted read directions were extracted as complex-valued numbers and averaged for each contrast, respectively. This results in a temporal update rate of 11.5s for concomitantly acquired VASO and BOLD respectively (23s combined). This means, we combined 16 segments of 8 inversion-recovery periods to generate two pairs of BOLD and VASO contrasts respectively. This was done to maximize the CNR at the canonical temporal frequency of functional connectivity fluctuations: 0.01Hz.
Results and Discussion
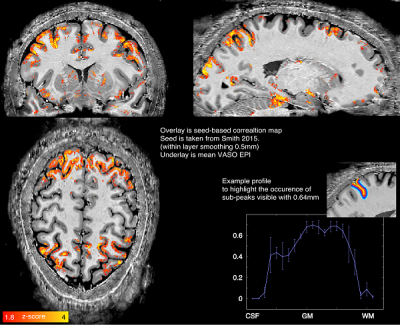
A seed for functional connectivity analysis was sourced from previous literature11. Figure 1 shows an example network from GLM with 0.5 mm within-layer smoothing12. The activation follows the gray matter ribbon, and shows indications of sub-layer-peaks in layer profiles (inset).The ability of layer-fMRI covering the entire brain at VASO at 0.64mm resolution is a step towards studying the interactions between distant regions of the brain and their involvement in modulating information processing via feedback activity as well as identify information flow between different brain areas by identifying signal at different depths in the cortex13. The presented initial results demonstrate that CBV based layer-fMRI can sample the neocortex which varies between 2 - 4mm thickness, with 3 - 6 voxels, respectively, which would reduce partial volume effects at boundaries from CSF and white matter and between adjacent layers.
Conclusion
In summary, whole brain imaging with CBV based layer-fMRI is achieved with 0.64mm isotropic resolution on the NexGen 7T scanner utilizing 64 channel Rx coil and Impulse head gradient. Further improvements in spatial resolution, TR and fMRI statistics may be possible with pulse sequence and hardware adaptations using a 128 channel Rx coil and additional sequence modifications14.Acknowledgements
This project is supported by the NIH BRAIN Initiative (R01MH111444, U01EB025162), 1R44MH129278. Samantha Ma is supported by Siemens Medical Solutions USA, Inc.
We thank Simon Robinson for discussions about most appropriate coil-combination methods in IcePat for appropriate estimation of Phase data.
We thank Rudiger Stirnberg for the use of the skipped-CAIPI 3D EPI sequence.
References
1. Olman CA, Harel N, Feinberg DA, He S, Zhang P, Ugurbil K, Yacoub E. Layer-specific fMRI reflects different neuronal computations at different depths in human V1. PLoS One 2012;7(3):e32536.
2. Zimmermann J, Goebel R, De Martino F, van de Moortele PF, Feinberg D, Adriany G, Chaimow D, Shmuel A, Ugurbil K, Yacoub E.Mapping the organization of axis of motion selective features in human area MT using high-field fMRI. PLoS One 2011;6(12):e28716.
3. Huber L, Handwerker DA, Jangraw DC, Chen G, Hall A, Stüber C, Gonzalez-Castillo J, Ivanov D, Marrett S, Guidi M, Goense J, Poser BA, and Bandettini PA. 2017. “High-Resolution CBV-FMRI Allows Mapping of Laminar Activity and Connectivity of Cortical Input and Output in Human M1.” Neuron 96(6):1253-1263.e7.
4. Feinberg, DA, Vu, AT, Beckett, AJS (2018). Pushing the limits of ultra-high resolution human brain imaging with SMS-EPI demonstrated for columnar level fMRI. Neuroimage, 164, 155-163.
5. Gunamony S, and Feinberg DA "An 8-channel transmit 64-channel receive compact head coil for Next Gen 7T scanner with head gradient insert." ISMRM 2022
6. Poser, BA, Koopmans PJ, Witzel, TL, Wald L, Barth M (2010) “Three Dimensional Echo-Planar Imaging at 7 Tesla.” Neuroimage. 2010 May 15;51(1):261-6
7. Stirnberg, R, and Stöcker T (2021). “Segmented K-space Blipped-controlled Aliasing in Parallel Imaging for High Spatiotemporal Resolution EPI.” Magnetic Resonance in Medicine 85(3):1540–51. doi: 10.1002/mrm.28486.
8. Feinberg DA, Torrisi S Beckett AJS, Stirnberg R, Stöcker T, Ehses P, Huber R. “Sub-0.1 microliter CBV fMRI on the Next Generation 7T scanner” ISMRM 2022.
9. Renzo Huber, Rüdiger Stirnberg , David A Feinberg, Samantha J Ma, Philipp Ehses, Omer Faruk Gulban, Jonathan R Polimeni, Kenshu Koiso, Emily Ma, Alexander JS Beckett, Tony Stöcker, Peter Bandettini, Benedikt A Poser “Low spatial-frequency ripple artifacts in layer-fMRI EPI: Identification, cause, and mitigation strategies with Dual-polarity readout.” ISMRM 2023
10. Stirnberg R, Deistung A, and Stöcker T. T2*-weighted dual-polarity skipped-CAIPI 3D-EPI: 400 microns isotropic whole-brain QSM at 7 Tesla in 6 minutes. #0594, ISMRM, 2022,
11. Smith, Stephen M., Peter T. Fox, Karla L. Miller, David C. Glahn, P. Mickle Fox, Clare E. Mackay, Nicola Filippini et al. "Correspondence of the brain's functional architecture during activation and rest." Proceedings of the national academy of sciences 106, no. 31 (2009): 13040-13045.
12. Blazejewska, A. I., Fischl, B., Wald, L. L., & Polimeni, J. R. (2019). Intracortical smoothing of small-voxel fMRI data can provide increased detection power without spatial resolution losses compared to conventional large-voxel fMRI data. Neuroimage, 189, 601-614. doi:10.1016/j.neuroimage.2019.01.054
13. Huber, Laurentius (Renzo), Emily S. Finn, Yuhui Chai, Rainer Goebel, Rüdiger Stirnberg, Tony Stöcker, Sean Marrett, Kamil Uludag, Seong-Gi Kim, SoHyun Han, Peter A. Bandettini, and Benedikt A. Poser. 2021. “Layer-Dependent Functional Connectivity Methods.” Progress in Neurobiology 207:101835. doi: 10.1016/j.pneurobio.2020.101835.
14. Park, Torrisi, Beckett, Vu, Feinberg “High Resolution fMRI: 3D EPI Temporal Random Walk Encoding on Nex-Gen 7T Scanner” OHBM 2022
Figures