0618
Detecting high temporal laminar-specific responses with line-scanning fMRI in awake mice1Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Harvard Medical School, Massachusetts General Hospital, Charlestown, MA, United States, 2School of Traditional Chinese Medicine, Southern Medical University, Guangzhou, China, 3Department of Neuroscience, Boston University, Boston, MA, United States
Synopsis
Keywords: fMRI (task based), fMRI
The 2D line-scanning fMRI has been used to map laminar BOLD onset and single-vessel vasodynamic changes. Lately, this method has been applied to map ultra-fast T2*-weighted MRI signal changes, directly coupled to the multi-unit activity in anesthetized mice with 5ms TR. Here, we have adjusted the 1D line-scanning fMRI method to test it for awake mice. The current setup enables the detection of evoked BOLD fMRI signals in the visual cortex following 4s (3 Hz, 20ms) light exposure. It provides the proof-of-concept to further examine the ultra-fast MRI signals directly linked to neuronal activity.Introduction
Laminar-specific fMRI provides an opportunity to investigate circuit-specific neuronal activity by measuring dynamic hemodynamic responses such as the blood-oxygen-level-dependent (BOLD)(1, 2). Previously, FLASH-based 1D line-scanning fMRI method has been developed with high temporal (50 ms) and spatial (50 um) resolution to characterize the fMRI onset time in anesthetized rats(3). Lately, FLASH based 2D line-scanning fMRI method was applied to directly map neuronal activity in anesthetized mice at high temporal (5 ms) and spatial (220 um) resolution based on the early work by Silva et al(4) and Yu et al(5), despite the underlying mechanism remains ambiguous. Here, we aim to establish the 1D line-scanning method to better elucidate laminar-specific fMRI signals with high temporal (up to 50 ms) and laminar spatial (25 um) resolution in awake mice. This work can offer an ultra-fast sampling rate without the interference of aliasing issues of the 2D line-scanning method, providing a potential solution to verify the mechanism of the previously reported ultrafast MRI signals for neuronal activity.Methods
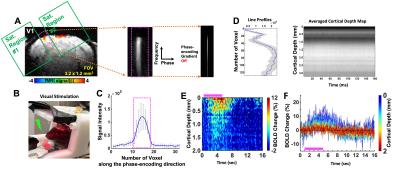
FLASH based line-scanning fMRI datasets were acquired in one awake mouse (7 trials) with a 14T scanner using a homemade transceiver head-post surface coil with a 10 mm diameter (Fig. 1B). The line-scanning method was applied by combining 2 saturation RF pulses to dampen the MR signal outside the regions of interest (ROI), i.e. the primary visual cortex (V1) (Fig. 1A). Readout direction was oriented perpendicular to the V1 cortical layers based on a FLASH based anatomical image in a coronal plane. The following parameters were used for the acquisition, TR/TE/FA 100/6 ms/30°, 50/4 ms/20°, TA 8 min 32 sec for each trial, slice thickness 1.5 mm, FOV 3.2 x 1.2 mm2 and matrix 128 x 32. The phase-encoding gradient was turned off to perform line-scanning acquisition.Functional activation was identified by performing a visual flash stimulation task (3Hz, 4s, pulse width 20ms), followed by 1s pre-stimulation, 4s visual stimulation and 11s post-stimulation with a total of 16 seconds for 8 min 32 sec (32 epochs). Visual stimulation consists of two LED lights flashing (488nm 5Hz, 530nm, 5.1Hz with 5ms offset, duration 20ms). All signal processing and analysis are implemented in Matlab software (Mathworks, Natick, MA). Averaged time course and percentage change map were calculated for averaging every 16 seconds with the whole time course (512 seconds).
Results
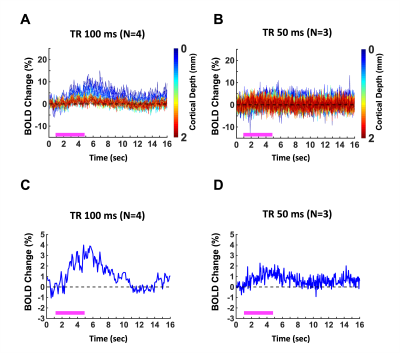
The laminar-specific BOLD-fMRI signals were detected with the line-scanning method in awake mice at V1 (Fig. 1). To identify activated regions upon visual stimulation, the EPI-fMRI BOLD map was first created (Fig. 1A). The line profiles of MRI signal intensity were shown to demonstrate the saturation performance (Fig. 1C). Fig. 1D shows averaged line profiles across cortical depth, which allows identifying cortical surface. Fig. 1E/F presents the averaged BOLD percentage-change map and time courses across cortical layers (0 – 2mm) with a representative single trial from one animal. Meanwhile, we also plot the BOLD time courses with the sampling rate at 100ms and 50ms, showing the BOLD responses at the superficial cortical regions (Fig. 2A/B). Furthermore, to identify hemodynamic response functions at 100ms and 50ms, the BOLD responses with averaged voxel were shown in Fig. 2C/D.Discussions and Conclusions
This work demonstrates the proof-of-concept of ultra-fast sampling of fMRI signals cross activated brain regions in awake mice. The usage of awake mice for preclinical fMRI enables function-behavioral mechanistic studies. Also, ultra-fast sampling enables the spectral analysis of multiple noise sources, e.g. heart beats, respiration, and other vibrational noises. It is also feasible to push TR to be close to 5ms by adjusting the acquisition bandwidth and minimizing the saturation slice pulse duration. Eventually, this line-scanning method could provide a critical tool for sampling fast MRI signals related to brain function.Acknowledgements
This research was funded by NIH funding (RF1NS113278, RF1NS124778, R01NS122904, R01NS120594, R21NS121642), NSF grant 2123971, and the S10 instrument grant (S10 MH124733–01) to Martino’s Center.References
1. Choi S, Chen Y, Zeng H, Biswal B, Yu X. Laminar-specific interhemispheric connectivity mapping with bilateral line-scanning fMRI. BioRxiv. 2021. doi: doi.org/10.1101/2021.03.08.433876
2. Choi S, Zeng H, Chen Y, Sobczak F, Qian C, Yu X. Laminar-Specific Functional Connectivity Mapping with Multi-Slice Line-Scanning fMRI. Cereb Cortex. Feb 2 2022;doi:10.1093/cercor/bhab497
3. Yu X, Qian C, Chen DY, Dodd SJ, Koretsky AP. Deciphering laminar-specific neural inputs with line-scanning fMRI. Nature methods. 2014;11(1):55-8. Epub 2013/11/19. doi: 10.1038/nmeth.2730. PubMed PMID: 24240320; PMCID: PMC4276040.
4. Silva AC, Koretsky AP. Laminar specificity of functional MRI onset times during somatosensory stimulation in rat. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(23):15182-7. doi: 10.1073/pnas.222561899. PubMed PMID: 12407177; PMCID: 137564.
5. Yu X, He Y, Wang M, Merkle H, Dodd SJ, Silva AC, Koretsky AP. Sensory and optogenetically driven single-vessel fMRI. Nature methods. 2016;13(4):337-40. doi: 10.1038/nmeth.3765. PubMed PMID: 26855362.
Figures