0596
Vertical MRI systems may offer a safer platform with substantially reduced RF heating in adult and pediatric patients with CIEDs1Biomedical Engineering, Northwestern University, Evanston, IL, United States, 2Radiology, Northwestern University, Chicago, IL, United States, 3Cardiology, Ann and Robert H. Lurie Children's Hospital of Chicago, Northwestern University Feinberg School of Medicine, Chicago, IL, United States, 4Athinoula A. Martinos Center for Biomedical Engineering, Charlestown, MA, United States
Synopsis
Keywords: Safety, Cardiovascular, Implants
MRI is restricted for patients with cardiac implantable electronic devices (CIEDs)—especially children with epicardial systems—due to potential radiofrequency (RF) heating of the tissue around the lead. Here, we present the first assessment of RF heating of epicardial and endocardial CIEDs with varying lead lengths in a vertical MRI system, where we found up to a 78-fold reduction in the simulated maximum specific absorption rate (SAR) compared to a horizontal coil. Reduction in the maximum 0.1g-averaged SAR in the vertical coil was consistent for leads with various internal wire geometries and electrical lengths.Introduction
Approximately 75% of patients with cardiac implantable electronic devices (CIEDs) will have clinical indications for MRI during their lifetime1. Radiofrequency (RF) induced localized tissue heating at the lead-tip is a well-known safety risk, restricting accessibility to MRI for these patients2,3. While epicardial systems are the most prevalent implants in pediatric patients, the FDA has not approved an epicardial MR-conditional CIED. Recent studies on the application of vertical MRI systems reported significantly reduced RF heating of deep brain stimulation implants in adults compared to horizontal MRI systems4,5. In this work, we present the first study evaluating the RF heating of epicardial and endocardial CIEDs with varying lead lengths and clinically realistic trajectories implanted in adults and children in a vertical, open-bore MRI system compared to a conventional horizontal MRI system.Methods
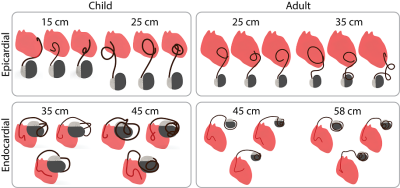
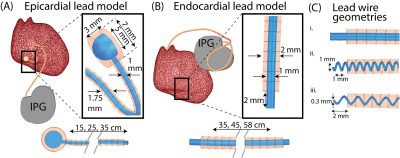
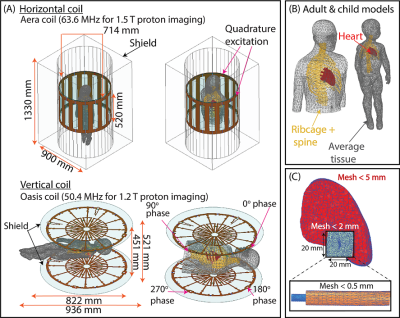
Clinically relevant lead trajectories: The lead’s trajectory, position in the body, and length have nontrivial effects on the variations in the magnitude of RF heating during MRI6,7. Thus, models of epicardial and endocardial leads with clinically relevant trajectories were created based on X-ray images of adult and pediatric patients with CIEDs, totaling 24 unique trajectories (Figure 1).Electromagnetic (EM) simulations: Full-wave EM simulations (ANSYS Electronic Desktop 2021 R1 HFSS, ANSYS, Canonsburg, PA) were performed to compare the specific absorption rate (SAR) amplification in each MRI system. Models of the 15, 25, and 35 cm epicardial leads mimicking the Medtronic CapSure® EPI 4965 leads were created (Figure 2A). Additionally, models were constructed for the 35, 45, and 58 cm endocardial leads mimicking the CapSureFix Novus MRI™ SureScan™ bipolar 5076 leads (Figure 2B). The internal core wires of the epicardial and endocardial leads were made of platinum-iridium (σ=4 x 106 S/m) embedded within urethane insulation (σ=0 S/m, 𝜀r=3.5). Models of the full pacemaker system inclusive of the lead and the implantable pulse generator (IPG)—mimicking the Medtronic Azure™ XT DR MRI SureScan™—were positioned in body models of an average adult male and a 29-month-old child, each consisting of three tissue classes: the heart (σ=0.7 S/m, 𝜀r=80), cancellous bone for the ribs and spine (σ=0 .16 S/m, 𝜀r=30.9), and average tissue for the remainder of the body model (σ=0.47 S/m, 𝜀r=80) (Figure 3). Vendor-specific models of a high-pass, radial planar 12-rung birdcage coil tuned to 50.4 MHz and a high-pass 16-rung horizontal birdcage coil tuned to 63.6 MHz were used in the simulations (Figure 3). The maximum of the 0.1g-averaged SAR (referred to as 0.1gSARmax) was quantified at the lead-tip where the input voltage applied to each coil was adjusted to generate a mean B1+ of 4 μT on a transverse plane at the center of the coil. A one-tailed Wilcoxon signed rank test was performed in the R software in RStudio (version 4.2.1) to compare the 0.1gSARmax in each MRI system. Statistical significance was established for p < 0.05.
Apparent versus electrical lead lengths: Additional simulations were performed to evaluate the effect of the lead’s length and internal geometry on SAR amplification. For an example trajectory, the core wire was modeled as a straight wire (electrical length=35 cm) or helical wire with pitches of 2 mm (electrical length=67 cm) and 1 mm (electrical length=120 cm) (Figure 2C). The remaining simulation details are as previously described.
Results
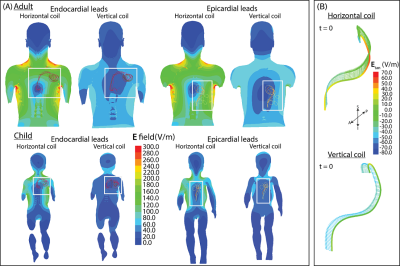
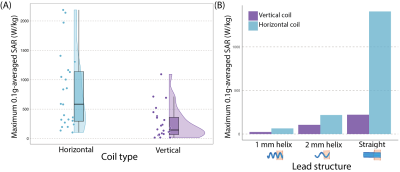
The magnitude of the incident electric field was calculated in the simulations of the adult and child body models without any CIEDs present. The electric field values were overall lower in the vertical coil and specifically in the region around the epicardial and endocardial devices (Figure 4A).With a mean B1+=4 μT, 0.1gSARmax was significantly lower in the 1.2 T vertical coil compared to the 1.5 T horizontal coil across lead lengths, trajectories, and type (p=3.79x10-5). The mean ± standard deviation of 0.1gSARmax was 254±273 W/kg and 794±641 W/kg in the vertical and horizontal coils, respectively. Figure 5A compares the distribution of 0.1gSARmax in the two MRI coils; 0.1gSARmax was 14-1,093 W/kg and 103–2,187 W/kg in the vertical and horizontal coils, respectively. Figure 5B illustrates that 0.1gSARmax is consistently lower in the vertical coil despite the different total electrical lengths of the lead; 0.1gSARmax was 23-220 W/kg and 63–1,407 W/kg in the vertical and horizontal coils, respectively.
Discussion and Conclusion
We presented the first results demonstrating significantly reduced SAR of epicardial and endocardial CIEDS in a vertical MRI system compared to a horizontal system. We compared the SAR amplification of leads with lengths of 15-58 cm in models based on pediatric and adult patients. Across 24 trajectories, there was up to a 78-fold reduction in SAR amplification. Additional simulations showed that the 0.1gSARmax was substantially reduced in the vertical coil compared to the horizontal coil across different geometries of the internal wire of the lead, indicating that such reduction in SAR may be possible for CIEDs with different total electrical lengths. While future work includes phantom experiments with actual CIEDs to supplement these findings, vertical open-bore MRI can potentially provide a safe method to image patients with CIEDs, specifically children with epicardial CIEDs.Acknowledgements
This work was supported by NIH grants R03EB029587 and T32EB025766.References
1. Kalin R, Stanton MS. Current clinical issues for MRI scanning of pacemaker and defibrillator patients. Pacing Clin Electrophysiol 2005;28(4):326-328.
2. Balmer C, Gass M, Dave H, et al. Magnetic resonance imaging of patients with epicardial leads: in vitro evaluation of temperature changes at the lead tip. Journal of Interventional Cardiac Electrophysiology 2019;56(3):321-326.
3. Indik JH, Gimbel JR, Abe H, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Heart rhythm 2017;14(7):e97-e153.
4. Golestanirad L, Kazemivalipour E, Lampman D, et al. RF heating of deep brain stimulation implants in open‐bore vertical MRI systems: A simulation study with realistic device configurations. Magnetic resonance in medicine. 2020; 83(6):2284-92.
5. Kazemivalipour E, Bhusal B, Vu J, et al. Vertical open‐bore MRI scanners generate significantly less radiofrequency heating around implanted leads: A study of deep brain stimulation implants in 1.2 T OASIS scanners versus 1.5 T horizontal systems. Magnetic resonance in medicine. 2021;86(3):1560-72.
6. Mattei E, Triventi M, Calcagnini G, et al. Complexity of MRI induced heating on metallic leads: Experimental measurements of 374 configurations. Biomed Eng Online 2008;7:11.
7. Nordbeck P, Weiss I, Ehses P, et al. Measuring RF-induced currents inside implants: Impact of device configuration on MRI safety of cardiac pacemaker leads. Magn Reson Med 2009;61:570–8.
Figures