0591
Brain and cervical spinal cord myelination and age-related changes in adulthood: a preliminary study based on ihMTsat and T1 relaxometry mapping1Aix-Marseille Univ, CNRS, CRMBM, Marseille, France, 2APHM, Hopital Universitaire Timone, CEMEREM, Marseille, France, 3Aix-Marseille Univ, Université Gustave Eiffel, LBA, Marseille, France, 4iLab-Spine International Associated Laboratory, Montreal, Canada, Marseille, France, 5Siemens Healthcare SAS, Saint-Denis, France
Synopsis
Keywords: Neurodegeneration, Aging
Inhomogeneous Magnetization Transfer (ihMT) has shown to be a biomarker of myelin with enhanced specificity as compared to conventional MT. To compensate for B1+ and T1 effects, a 3D ihMT-RAGE sequence with an ihMTsat framework has recently been proposed.
In this study, ihMTsat was used in combination with an optimized MP2RAGE sequence to study both brain and spinal cord adulthood aging process.
The ihMTsat and R1=1/T1 metrics followed an inverse U-shaped evolution with age in different ROIs, from which a maturation age was extracted. A multiparametric (R1,ihMTsat) voxel-wise analysis revealed significant age effect in the microstructure of different cord tracts.
Introduction
A wide range of MR techniques has been developed in the past decades to provide sensitive and/or specific biomarkers of myelin and subsequently investigate myelin impairments occurring in various neurodegenerative diseases1 and normal aging2,3.Among them, the recently developed inhomogeneous Magnetization Transfer (ihMT)4 technique has attracted attention thanks to increased specificity to myelinated tissue5,6.
Nevertheless, the standard ihMT Ratio (ihMTR) derived from ihMT sequences can be biased by B1+ inhomogeneity and T1 relaxation effects7–9. To compensate from these effects, a strategy inspired by Helms et al10 was recently developed to derive a T1- and B1+- unbiased ihMTsat parameter11,12, and applied to study the cortical myeloarchitecture7.
In this study and for the first time, an ihMTsat approach, optimized for both brain and cervical spinal cord (cSC) imaging, was combined with an optimized MP2RAGE T1 mapping13,14 to study age-related changes in different regions of the human brain and cSC.
Materials & Methods
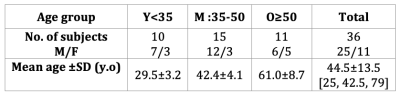
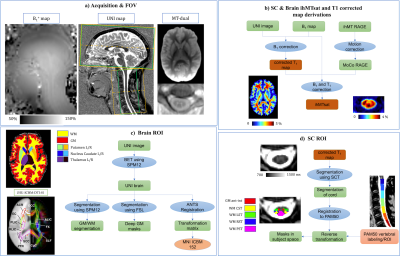
Thirty-six adult healthy subjects of different age ranges (younger (Y), middle-aged (M), and older (O), cf. Table 1) were scanned with a 3T MR system (MAGNETOM Vida, Siemens Healthcare, Germany) and a 20-channel head and neck coil.The protocol included: a prototype 3D ihMT-RAGE sequence with a 2-mm isotropic / 0.9×0.9×10-mm3 resolution for brain/cSC, respectively (cf. Fig1.a), a 0.9-mm isotropic resolution T1 Magnetization Prepared 2 Rapid Acquisition Gradient Echo (MP2RAGE) sequence14 optimized for simultaneous brain and cSC13, and a B1+ mapping based on a pre-saturated turbo flash sequence16.
Post-processing & Statistical analysis
The main post-processing steps are depicted in Figure 1.b-d. Briefly, T1 data were corrected for B1+ inhomogeneity as in17. The corrected T1 map, B1+ map and the motion-corrected MT volumes, were then used to calculate the ihMTsat map, based on the strategy described in 7,15,18 and using a freely available post-processing pipeline (https://github.com/lsoustelle/ihmt_proc).Quantification of T1 and ihMTsat metrics in the subject space were performed using the ROI masks from PAM5019 on SC and JHU WM tracts20,21 and MNI parcellation maps22,23 on brain. To investigate the evolution of metrics with age, a quadratic regression was used separately to fit T1 and ihMTsat values as a function of the subjects’ age, as performed in previous studies with R1 and DTI metrics24,25. A “maturation” age was derived from the fitted curve as the age at maximal ihMTsat (respectively minimal T1).
To further analyze the maturation of different tissues in the middle-aged group, R1 (=1/T1) and ihMTsat maps were used in voxel-wise multi-variate analyses using Non-Parametric Combination (NPC) tool26 from PALM (version alpha119) using 5000 permutations (p-value<0.05 for significance).
Results & Discussion
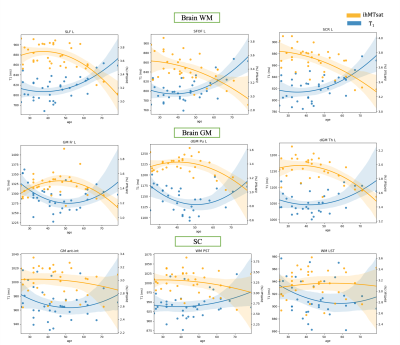
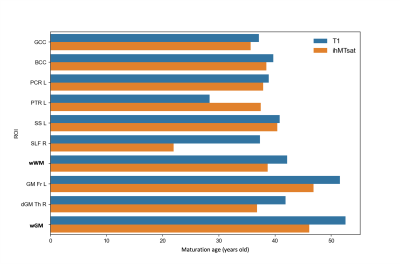
Examples of the quadratic fit for T1 and ihMTsat on different ROIs are illustrated in figure 2. The T1 values (blue curve) follow a U-shape trend with age (decreasing to a minimum and then increasing), in agreement with previous literature24 on brain. The ihMTsat values (orange curve) follow an inverse U-shape, as similarly observed for Fractional Anisotropy in DTI measurements25,27 on brain. Although similar trends for T1 and ihMTsat were observed for SC, none of the SC ROI regressions reached the significance level. To our knowledge, the quadratic evolution for quantitative metrics on SC has never been reported.The heterogeneity of the aging process and maturation across different regions is illustrated on Figure 3. The ihMTsat (resp T1) “maturation” ages were found in average as 38.6 (resp 42.1) years for brain whole WM, and 46.1 (resp 52.2) for brain whole GM (see fig. 3). In all GM ROIs, maturation age obtained from T1 was higher than ihMTsat, presumably due to concomitant sensitivity of T1 to other factors such as iron deposition that further reduces the metric value. This observation is consistent with the contrast fraction (~36% vs. 10%) that comes from iron in GM vs. WM28.
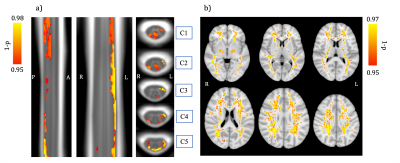
Finally, PALM analysis results combining R1 and ihMTsat values and comparing the middle-aged group to the younger and older ones using the WM mask are shown in Figure 4. Significant differences were observed in various regions such as PCR, SLF, PTR, and SS in brain WM, as well as PST and LST (especially left tracts) in cSC, whereas univariate analyses (with either modality) led to no significant clusters (data not shown). Interestingly, significant clusters in cSC WM, especially in sensory pathways, were demonstrated, which could indicate the presence of a maturation process in the cord, as observed in the brain. To the best of our knowledge, this has never been reported in the literature before.
No significant clusters were found in GM.
Conclusion
The ihMTsat metric was used for the first time to investigate aging in healthy adults’ brain and cSC. Parametric microstructural characterization of brain and SC using T1 and ihMT showed a lifespan quadratic evolution of brain WM and GM but not of SC. However, voxel-wise multiparametric approach combining the two parameters showed significant differences in WM bundle microstructure for middle-aged subjects compared to younger and older subjects both in brain and SC, suggesting a similar maturation and aging process in SC as in brain. This database is intended to be completed over time and made available for future studies investigating neurodegenerative pathologies.Acknowledgements
This work was performed within a laboratory member of France Life Imaging network (grant ANR-11-INBS-0006) and was supported by the Institut Carnot Star, the ARSEP Foundation (Fondation pour l’Aide à la Recherche sur la Sclérose en Plaques) and the CNRS (Centre National de la Recherche Scientifique). The project also received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No713750, with the financial support of the Regional Council of Provence-Alpes-Côte d’Azur. This work received support from the French government under the France 2030 investment plan, as part of the Initiative d'Excellence d'Aix-Marseille Université - A*MIDEX.References
1. Laule C, Vavasour IM, Kolind SH, et al. Magnetic resonance imaging of myelin. Neurotherapeutics. 2007;4(3):460-484. doi:10.1016/j.nurt.2007.05.004 SMASH
2. Taso M, Girard OM, Duhamel G, et al. Tract-specific and age-related variations of the spinal cord microstructure: A multi-parametric MRI study using diffusion tensor imaging (DTI) and inhomogeneous magnetization transfer (ihMT). NMR Biomed. 2016;29(6):817-832. doi:10.1002/nbm.3530 SMASH
3. Hill RA, Li AM, Grutzendler J. Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat Neurosci. 2018;21(5):683-695. doi:10.1038/s41593-018-0120-6 SMASH
4. Varma G, Duhamel G, De Bazelaire C, Alsop DC. Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin. Magn Reson Med. 2015;73(2):614-622. doi:10.1002/mrm.25174 SMASH
5. Duhamel G, Prevost VH, Cayre M, et al. Validating the sensitivity of inhomogeneous magnetization transfer (ihMT) MRI to myelin with fluorescence microscopy. Neuroimage. 2019;199(May):289-303. doi:10.1016/j.neuroimage.2019.05.061 SMASH
6. Alsop DC, Ercan E, Girard OM, et al. Inhomogeneous magnetization transfer imaging: Concepts and directions for further development. NMR Biomed. August 2022. doi:10.1002/nbm.4808 SMASH
7. Munsch F, Varma G, Taso M, et al. Characterization of the cortical myeloarchitecture with inhomogeneous Magnetization Transfer imaging (ihMT). Neuroimage. 2020;225(October 2020):117442. doi:10.1016/j.neuroimage.2020.117442 SMASH
8. Forodighasemabadi A, Troalen T, Soustelle L, Duhamel G, Girard O, Callot V. Towards minimal T1 and B1 contributions in cervical spinal cord inhomogeneous magnetization transfer imaging. In: Proceedings 29th Scientific Meeting, International Society for Magnetic Resonance in Medicine. ; 2020:p.1175.
9. Varma G, Munsch F, Girard OM, Duhamel G, Alsop DC. An inhomogeneous magnetization transfer ( ihMT ) quantification method robust to B1 and T1 variations in magnetization prepared acquisitions. In: Proceedings 27th Scientific Meeting, International Society for Magnetic Resonance in Medicine. ; 2019:p.4911.
10. Helms G, Dathe H, Kallenberg K, Dechent P. High-resolution maps of magnetization transfer with inherent correction for RF inhomogeneity and T1 relaxation obtained from 3D FLASH MRI. Magn Reson Med. 2008;60(6):1396-1407. doi:10.1002/mrm.21732 SMASH
11. Varma G, Munsch F, Burns B, et al. Three‐dimensional inhomogeneous magnetization transfer with rapid gradient‐echo (3D ihMTRAGE) imaging. Magn Reson Med. 2020;(April):mrm.28324. doi:10.1002/mrm.28324 SMASH
12. Rowley CD, Campbell JSW, Wu Z, et al. A model-based framework for correcting B1+ inhomogeneity effects in magnetization transfer saturation and inhomogeneous magnetization transfer saturation maps. Magn Reson Med. 2021;86(4):2192-2207. doi:10.1002/mrm.28831 SMASH
13. Forodighasemabadi A, Rasoanandrianina H, El Mendili MM, Guye M, Callot V. An optimized MP2RAGE sequence for studying both brain and cervical spinal cord in a single acquisition at 3T. Magn Reson Imaging. 2021;84(September):18-26. doi:10.1016/j.mri.2021.08.011 SMASH
14. Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage. 2010;49(2):1271-1281. doi:10.1016/j.neuroimage.2009.10.002 SMASH
15. Forodighasemabadi A, Baucher G, Soustelle L, et al. Spinal cord and brain tissue impairments as long-term effects of rugby practice? An exploratory study based on T1 and ihMTsat measures. NeuroImage Clin. 2022;35:103124. doi:10.1016/j.nicl.2022.103124 SMASH
16. Chung S, Kim D, Breton E, Axel L. Rapid B1+ mapping using a preconditioning RF pulse with turboFLASH readout. Magn Reson Med. 2010;64(2):439-446. doi:10.1017/S1355771817000310 SMASH
17. Massire A, Taso M, Besson P, Guye M, Ranjeva JP, Callot V. High-resolution multi-parametric quantitative magnetic resonance imaging of the human cervical spinal cord at 7T. Neuroimage. 2016;143:58-69. doi:10.1016/j.neuroimage.2016.08.055 SMASH
18. Helms G, Dathe H, Kallenberg K, Dechent P. High-resolution maps of magnetization transfer with inherent correction for RF inhomogeneity and T1 relaxation obtained from 3D FLASH MRI. Magn Reson Med. 2008;60(6):1396-1407. doi:10.1002/mrm.21732 SMASH
19. De Leener B, Fonov VS, Collins DL, Callot V, Stikov N, Cohen-Adad J. PAM50: Unbiased multimodal template of the brainstem and spinal cord aligned with the ICBM152 space. Neuroimage. 2018;165(July 2017):170-179. doi:10.1016/j.neuroimage.2017.10.041 SMASH
20. Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570-582. doi:10.1016/j.neuroimage.2007.12.035 SMASH
21. Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336-347. doi:10.1016/j.neuroimage.2007.07.053 SMASH
22. Fonov V, Evans A, McKinstry R, Almli C, Collins D. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Neuroimage. 2009;47:S102. doi:10.1016/s1053-8119(09)70884-5 SMASH
23. Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54(1):313-327. doi:10.1016/j.neuroimage.2010.07.033 SMASH
24. Yeatman JD, Wandell BA, Mezer AA. Lifespan maturation and degeneration of human brain white matter. Nat Commun. 2014;5(1):1-12. doi:10.1038/ncomms5932 SMASH
25. Hasan KM, Kamali A, Abid H, Kramer LA, Fletcher JM, Ewing-Cobbs L. Quantification of the spatiotemporal microstructural organization of the human brain association, projection and commissural pathways across the lifespan using diffusion tensor tractography. Brain Struct Funct. 2010;214(4):361-373. doi:10.1007/s00429-009-0238-0 SMASH
26. Winkler AM, Webster MA, Brooks JC, Tracey I, Smith SM, Nichols TE. Non-parametric combination and related permutation tests for neuroimaging. Hum Brain Mapp. 2016;37(4):1486-1511. doi:10.1002/hbm.23115 SMASH
27. Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60(1):340-352. doi:10.1016/j.neuroimage.2011.11.094 SMASH
28. Stüber C, Morawski M, Schäfer A, et al. Myelin and iron concentration in the human brain: A quantitative study of MRI contrast. Neuroimage. 2014;93(P1):95-106. doi:10.1016/j.neuroimage.2014.02.026 SMASH
Figures