0585
Shared and differed functional connectivity abnormalities for default mode network in mild cognitive impairment and Alzheimer’s disease1Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu 610041, Sichuan, P.R. China., Chengdu, China
Synopsis
Keywords: Neurodegeneration, Brain Connectivity
We performed a meta-analysis to investigate shared and different alterations of resting-state functional connectivity (rsFC) for default mode network (DMN) in mild cognitive impairment (MCI) and Alzheimer’s disease (AD). We found both patients with MCI and AD showed hypoconnectivity within DMN in bilateral medial prefrontal cortex (mPFC)/anterior cingulate cortex (ACC) and precuneus/posterior cingulate gyrus (PCC), indicating possibly shared neuropathologic mechanism underlying common cognitive dysfunction. Moreover, we identified distinct alterations of rsFC for DMN in some brain regions, which suggested potential imaging markers to distinguish the two diseases.Introduction
AD is the most common form of dementia 1, while MCI represents a transitional stage between healthy aging and dementia 2. Both of them are characterized by impairments of cognitive function involving multiple domains 3. Considerable evidence found by magnetic resonance imaging studies suggests that cognitive dysfunction in these patients is associated with disrupted rsFC, especially DMN 4. While some meta-analyses suggest decreased DMN rsFC in MCI and AD 5-7, their differences and commonalities have not been elucidated. We therefore conduct a meta-analysis to establish shared and different alterations of rsFC for DMN in MCI and AD, which can deepen our understanding of their pathophysiology and facilitate intervention at the early stage.Methods
We searched in the PubMed, Web of Science, and Embase databases for relevant original articles published through 18 September 2022. Search keywords included: “mild cognitive impairment” or “MCI” or “Alzheimer's disease” or “Alzheimer disease” or “Alzheimer's” or “dementia of the Alzheimer’s type” or “Alzheimer’s dementia” plus “fMRI” or “functional magnetic resonance imaging” or “rsfMRI” or “functional connectivity” or “resting state functional connectivity” plus “default mode network” or “DMN”. We included original studies using seed-based whole-brain analysis or independent component analysis to compare patients with MCI or AD with healthy controls (HCs) for aberrant rsFC for DMN. We conducted the meta-analysis using the anisotropic effect size version of the signed differential mapping (AES-SDM) software package (version 5.15) 8. First, separate meta-analyses were performed to identify rsFC alterations for DMN in MCI and AD relative to HCs. Next, a quantitative comparison between MCI and AD was performed. We used the default thresholds (voxel threshold P<0.005 with peak Z>1) with a cluster extent of 100 voxels. Then, a conjunction/disjunction analysis was performed to investigate shared/contrasting aberrant rsFC across patient groups utilizing the multimodal analysis in SDM, with a stringent probability threshold (P<0.0025) 9. Jackknife sensitivity, heterogeneity, and publication bias analysis were performed. We conducted meta-regression with mean age of patients, percentage of female patients, and Mini-Mental State Examination (MMSE) score as regressors using a threshold of 0.0005 10.Results
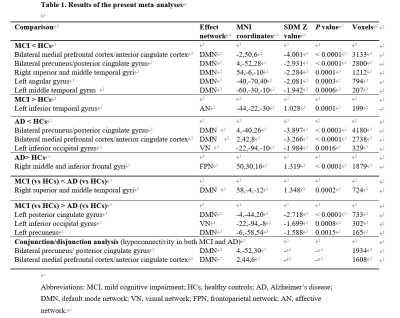
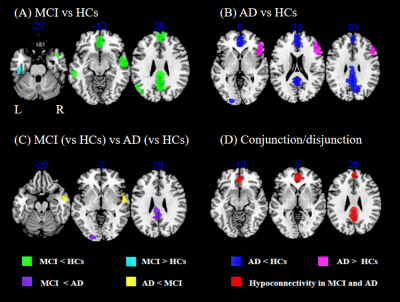
We included 31 MCI studies comprising 960 patients with MCI and 1084 HCs and 20 AD studies comprising 569 patients with AD and 661 HCs. Relative to HCs, patients with MCI showed hypoconnectivity between DMN and bilateral mPFC/ACC and precuneus/PCC, right superior and middle temporal gyri, left angular gyrus and left middle temporal gyrus (MTG), and hyperconnectivity between DMN and left inferior temporal gyrus (ITG) (Table 1 and Fig. 1A). Relative to HCs, patients with AD showed hypoconnectivity between DMN and bilateral precuneus/PCC and mPFC/ACC and left inferior occipital gyrus (IOG), and hyperconnectivity between DMN and right middle and inferior frontal gyri (Table 1 and Fig. 1B). Relative to AD, patients with MCI showed decreased DMN rsFC in right superior and middle temporal gyri, and increased rsFC between DMN and left PCC, left precuneus, and left IOG (Table 1 and Fig. 1C). The conjunction/disjunction analysis revealed that patients with MCI and AD had shared within-DMN-hypoconnectivity in bilateral mPFC/ACC and precuneus/PCC (Table 1 and Fig. 1D). Heterogeneity and publication bias were identified in AD studies for right middle and inferior frontal gyri (P=0.017). Neither mean age of patients, percentage of females, and MMSE score were significantly associated with rsFC alterations in MCI and AD.Discussion
We found patients with MCI and AD have shared within-DMN-hypoconnectivity in mPFC/ACC and precuneus/PCC, crucial regions for cognitive processes, including memory encoding and self-referential processes 11. Thus, shared hypoconnectivity in these regions may underlie shared cognitive impairements in MCI and AD, such as episodic memory disturbance and anosognosia 12, 13. Moreover, we found that decreased within-DMN rsFC in AD was more pronounced than in MCI in two smaller clusters (left PCC and precuneus), indicating disconnection in these regions deteriorates as the disease progresses. Compared to AD, MCI patients showed lesser DMN rsFC in right superior and middle temporal gyri, regions implicated in language cognition 14, 15. Since language deficits can be seen at the stage of MCI 16, these deficits in MCI might be related to abnormal connectivity in right superior and middle temporal gyri. Relative to MCI, AD patients exhibited reduced rsFC between DMN and IOG, a region affected late across AD evolution 17 and responsible for visuospatial function 18. Thus, this abnormality may underlie visuospatial dysfunction in AD. Specific rsFC alterations for DMN of each disease relative to their corresponding HCs were also found in left angular gyrus, left MTG (MCI less than HCs), and right middle and inferior frontal gyri (AD more than controls). These results can be served as potential neuroimaging markers to distinguish the two diseases. However, the finding that hyperconnectivity between DMN and right middle and inferior frontal gyri should be treated as caution and requires further verification since the heterogeneity and publication bias.Conclusion
In summary, our results suggested shared and different rsFC alterations for DMN in MCI and AD patients, providing evidence of shared neuropathologic mechanisms and potential neuroimaging biomarkers to distinguish the two diseases.Acknowledgements
No acknowledgment was found.References
1. McKhann, G.M., et al., The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement, 2011. 7(3): p. 263-9.
2. Mitchell, A.J. and M. Shiri-Feshki, Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand, 2009. 119(4): p. 252-65.
3. Albert, M.S., et al., The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement, 2011. 7(3): p. 270-9.
4. Nuttall, R., et al., Degradation in intrinsic connectivity networks across the Alzheimer's disease spectrum. Alzheimers Dement (Amst), 2016. 5: p. 35-42.
5. Badhwar, A., et al., Resting-state network dysfunction in Alzheimer's disease: A systematic review and meta-analysis. Alzheimers Dement (Amst), 2017. 8: p. 73-85.
6. Eyler, L.T., et al., Resting State Abnormalities of the Default Mode Network in Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. J Alzheimers Dis, 2019. 70(1): p. 107-120.
7. Wang, C., et al., Aberrant default mode network in amnestic mild cognitive impairment: a meta-analysis of independent component analysis studies. Neurol Sci, 2018. 39(5): p. 919-931.
8. Radua, J., et al., A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry, 2012. 27(8): p. 605-11.
9. Epstein, J.N., et al., Differential patterns of brain activation over time in adolescents with and without attention deficit hyperactivity disorder (ADHD) during performance of a sustained attention task. Neuropediatrics, 2009. 40(1): p. 1-5.
10. Radua, J., et al., Multimodal voxel-based meta-analysis of white matter abnormalities in obsessive-compulsive disorder. Neuropsychopharmacology, 2014. 39(7): p. 1547-57.
11. Sperling, R.A., et al., Functional alterations in memory networks in early Alzheimer's disease. Neuromolecular Med, 2010. 12(1): p. 27-43.
12. Mondragon, J.D., N.M. Maurits, and P.P. De Deyn, Functional Neural Correlates of Anosognosia in Mild Cognitive Impairment and Alzheimer's Disease: a Systematic Review. Neuropsychol Rev, 2019. 29(2): p. 139-165.
13. Soman, S.M., et al., Does resting state functional connectivity differ between mild cognitive impairment and early Alzheimer's dementia? J Neurol Sci, 2020. 418: p. 117093.
14. Briggs, R.G., et al., The Unique Fiber Anatomy of Middle Temporal Gyrus Default Mode Connectivity. Oper Neurosurg (Hagerstown), 2021. 21(1): p. E8-E14.
15. Yi, H.G., M.K. Leonard, and E.F. Chang, The Encoding of Speech Sounds in the Superior Temporal Gyrus. Neuron, 2019. 102(6): p. 1096-1110.
16. Khatoonabadi, A.R. and J. Masumi, Study protocol: Language profile in mild cognitive impairment: A prospective study. Med J Islam Repub Iran, 2019. 33: p. 53.
17. Weiler, M., et al., Default mode, executive function, and language functional connectivity networks are compromised in mild Alzheimer's disease. Curr Alzheimer Res, 2014. 11(3): p. 274-82.
18. Bogousslavsky, J., et al., Lingual and fusiform gyri in visual processing: a clinico-pathologic study of superior altitudinal hemianopia. J Neurol Neurosurg Psychiatry, 1987. 50(5): p. 607-14.
Figures