0584
Quantitative correlations of propagator metrics with phosphorylated tau and astrogliosis in chronic traumatic encephalopathy1Center for Neuroscience and Regenerative Medicine, Bethesda, MD, United States, 2The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, MD, United States, 3Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, MD, United States, 4Section on Critical Brain Dynamics, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, United States, 5Section on Quantitative Imaging and Tissue Sciences, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, United States, 6Biomedical Engineering, University of Arizona, Tucson, AZ, United States, 7Laboratory of Functional and Molecular Imaging, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD, United States, 8Multiscale Imaging and Integrative Biophysics Unit, National Institute on Aging, National Institutes of Health, Bethesda, MD, United States
Synopsis
Keywords: Neurodegeneration, Diffusion/other diffusion imaging techniques
Propagator metrics computed from high spatial resolution diffusion MRI data are correlated with histopathological assessments of phosphorylated tau (p-tau) and astrogliosis in tissue specimen with a diagnosis of Stage III/IV chronic traumatic encephalopathy (CTE). Region of interest based analysis showed significant correlations of p-tau with non-Gaussianity (NG) in deep cortical gray matter and of propagator anisotropy (PA) with astrogliosis in superficial cortical white matter. An unsupervised clustering approach with PA and NG as inputs is then used to segment MR data of tissue specimen into clusters to determine whether propagator metrics can be utilized to detect underlying pathology.Introduction
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease that is diagnosed and staged based on the localization and extent of phosphorylated tau (p-tau) pathology1. Although the identification of p-tau pathology remains the primary diagnostic criteria to distinguish CTE from other tauopathies, the hyperphosphorylated tau that accumulates in neurofibrillary tangles in cortical gray matter and perivascular regions is often accompanied by concomitant pathology such as astrogliosis. These features are currently identifiable post-mortem, making a non-invasive imaging approach valuable to provide an in vivo diagnosis of CTE. Diffusion MRI is sensitive to microstructural alterations in cortical tissue and tensor-based measurements in tissue specimen with CTE have demonstrated sensitivity to white matter disruption adjacent to accumulated sulcal p-tau2; however, a relationship between the diffusion signal and p-tau has yet to be determined. Mean apparent propagator (MAP) MRI is a clinically feasible diffusion MRI method capable of comprehensively and efficiently characterizing microstructure of complex biological media using metrics such as propagator anisotropy (PA) and non-Gaussianity (NG)3. The purpose of the current work is to identify correlations between MAP-MRI metrics and p-tau and reactive astrocytes identified using histological methods in tissue specimen with Stage III/IV CTE. We first use linear mixed effects analysis of regions of interest (ROI) with underlying pathology to identify suitable MAP-MRI metrics and input these metrics to a k-means clustering algorithm in order to determine whether MAP-MRI at high spatial resolution can be used to detect CTE pathology.Methods
Data for MAP-MRI estimation were derived from previously acquired diffusion data in ten tissue2 specimen with a confirmed diagnosis of Stage III/IV CTE. Briefly, high angular resolution diffusion data (TR/TE = 1400/30 ms) were collected across 202 noncollinear diffusion sensitized directions with a maximum b-value of 8,000 s/mm2 along with ten non-diffusion weighted (b = 0 s/mm2) volumes. For the purposes of this study, diffusion datasets were upsampled to have isotropic 250 µm spatial resolution and diffusion tensor (DTI) and MAP-MRI model fitting was performed in TORTOISE4. P-tau and reactive astrocytes were quantified in corresponding tissue sections stained with AT8 and GFAP2,5 respectively in ROIs selected by an investigator blinded to diffusion metrics. Correspondence of abundance of p-tau and astrogliosis with MAP-MRI metrics was assessed using a linear mixed effects (LME) analysis with inference testing performed using analysis of variance (lme command, R Development Core Team 2021). ROIs in deep cortical gray matter and superficial cortical white matter were analyzed separately, with specimen number as a random variable in the model to account for inter-specimen variability in post-mortem interval and tissue fixation period. Bonferroni’s method for multiple comparisons was used to adjust for false discovery. A linear regression analysis was performed for relationships found to be significant in the LME model. A voxel-based k-means clustering approach with enforced spatial contiguity was applied with metrics determined to be significant from the LME analysis and used four clusters to segment tissue specimen into classes hypothesized to correspond with (1) gray matter with pathology (2) gray matter without pathology (4) white matter with pathology (4) white matter without pathology.Results
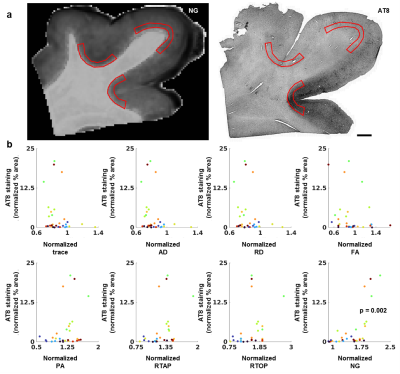
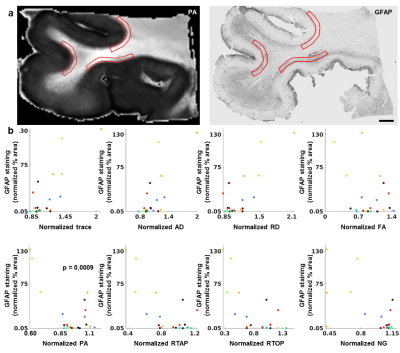
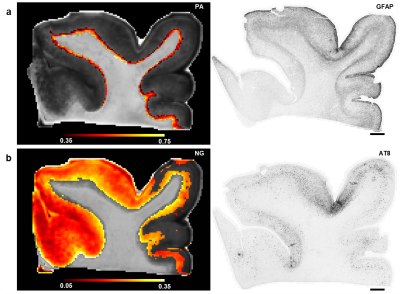
Figure 1 shows the relationships of p-tau with DTI and MAP-MRI metrics plotted by tissue specimen and the LME results that remained significant after correction for multiple comparisons. NG in deep cortical gray matter corresponded with regions of increased accumulation of p-tau (p = 0.002), and subsequent linear regression analysis showed that this correlation was modest and significant (R2 = 0.44, p = 6.0 x 10-5). The scatterplots of the relationship of astrogliosis with diffusion metrics in superficial cortical white matter are shown in Figure 2. The LME model determined that the relationship between PA and astrogliosis was significant (p = 0.0009) and linear regression results showed that the correlation was weak but significant (R2 = 0.15, p = 0.004). When NG and PA were used as inputs to the k-means clustering algorithm with four voxel classes, a cluster in superficial cortical white matter was consistently identified in the ten tissue specimen that corresponded with regions of dense GFAP staining. PA values in the identified cluster were generally reduced in contrast with voxels along the gray-white matter interface that were classified into a separate cluster. A second tissue class was also generated that primarily consisted of voxels in deep cortical gray matter, but also included voxels in superficial layers. Voxels within the tissue cluster generally had increased NG, however the values were more heterogenous across the layers of cortical gray matter and did not show a visually apparent correspondence with AT8 staining.Discussion
We show that regions with increased p-tau and astrogliosis corresponded with increased NG and PA respectively and were useful when segmenting tissue into clusters that identified areas of high pathology. In comparison to the tensor model, use of metrics derived from the full propagator show promising utility in detecting complex alterations that occur in tissue microstructure in tissue specimen with concomitant pathology.Acknowledgements
We are grateful to the donors and their families who were able to make this study possible. We would also like to thank the Boston University CTE Center in particular, Ann C. McKee, Thor Stein and Victor Alvarez for sample procurement and for providing diagnosis information regarding tissue specimen. We are grateful to Eric Hsu, and Shiran Hsu for their assistance in the histological processing of tissue specimen. Specimen imaging was performed at the Small-Animal Imaging Facility of the Mallinckrodt Institute of Radiology and the Center for Cellular Imaging at Washington University School of Medicine. Support for the data collection portion of this study was provided by NIH UO1 NS086659-02 (Overall P.I: A. McKee, Subproject 3 P.I: Brody). Support for data analysis was provided by the Center for Neuroscience and Regenerative Medicine Military TBI Fellowship (CNRM-92-11216), the CNRM Neuroradiology/Neuropathology Correlation/Integration Core (CNRM-89-9921), the Intramural Research Program (IRP) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number ZIAHD000266).
Disclaimer: The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of the Henry M. Jackson Foundation for the Advancement of Military Medicine Inc., Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, the Department of the Air Force, the Department of Defense, or the U.S. Government
References
1. A.C. McKee, N.J. Cairns, D.W. Dickson, R.D. Folkerth, C.D. Keene, I. Litvan, D.P. Perl, T.D. Stein, J.P. Vonsattel, W. Stewart, Y. Tripodis, J.F. Crary, K.F. Bieniek, K. Dams-O'Connor, V.E. Alvarez, W.A. Gordon, T.C. group, The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy, Acta Neuropathol 131(1) (2016) 75-86.
2. L. Holleran, J.H. Kim, M. Gangolli, T. Stein, V. Alvarez, A. McKee, D.L. Brody, Axonal disruption in white matter underlying cortical sulcus tau pathology in chronic traumatic encephalopathy, Acta Neuropathol 133(3) (2017) 367-380.
3. E. Ozarslan, C.G. Koay, T.M. Shepherd, M.E. Komlosh, M.O. Irfanoglu, C. Pierpaoli, P.J. Basser, Mean apparent propagator (MAP) MRI: a novel diffusion imaging method for mapping tissue microstructure, Neuroimage 78 (2013) 16-32.
4. Pierpaoli, C., Walker, L., Irfanoglu, M. O., Barnett, A., Basser, Chang, P.L-C., Koay, C., Pajevic, S., Rohde, G., Sarlls, J., Wu, M. in ISMRM 18th annual meeting.
5. E.T. Hsu, M. Gangolli, S. Su, L. Holleran, T.D. Stein, V.E. Alvarez, A.C. McKee, R.E. Schmidt, D.L. Brody, Astrocytic degeneration in chronic traumatic encephalopathy, Acta Neuropathol 136(6) (2018) 955-972.
Figures