0583
MRI detects macro- and microstructural changes to normal brain tissue following hemi-brain radiotherapy1Division of Informatics, Imaging and Data Sciences, Faculty of Biology, Medicine and Health, University of Manchester, UK, Manchester, United Kingdom, 2Division of Cancer Sciences, School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester, UK, Manchester, United Kingdom, 3Department of Radiotherapy Related Research, The Christie NHS Foundation Trust, Manchester, United Kingdom, Manchester, United Kingdom, 4Division of Cancer Sciences, School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester, UK, Manchester, UT, United Kingdom, 5Division of Pharmacy and Optometry, School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester, UK, Manchester, United Kingdom
Synopsis
Keywords: Neurodegeneration, Radiotherapy
Approximately 50-90% of patients that survive treatment for brain tumours experience dementia-like cognitive impairments. Here we use MRI and behavioural testing to assess longitudinal changes to brain macro- and microstructure and cognitive dysfunction following hemi-brain radiotherapy. We show shrinkage of cortical and hippocampal regions in the irradiated hemisphere, and expansion of cortical tissue in the non-irradiated hemisphere relative to non-irradiated control mice. Brain microstructure was also changed including increases in gray matter diffusivity, and decreases in white matter fractional anisotropy. Changes on MRI were accompanied by early and persistent deficits in novel object recognition.Introduction
Radiation-induced neurocognitive decline, such as reductions in processing speed, executive function, and verbal fluency have become more relevant toxicities in recent years as the number of long-term survivors following brain radiotherapy has increased1. Approximately 50-90% of patients who survive past 6 months experience dementia-like cognitive impairments2,3, significantly worsening quality of life.There is evidence from rodent and human studies of the detrimental effects of radiotherapy on the brain, such as brain atrophy, demyelination of white matter, and loss of dendritic spines in gray matter. To help establish tools to assess therapies that aim to ameliorate neurotoxicity, we have developed a longitudinal imaging and behavioural pipeline in mice. In this study, we applied this pipeline to track macrostructural, microstructural, and neurocognitive changes after single-dose hemi-brain radiotherapy.
Methods
Twenty male C57BL6 mice aged 6 months were randomised into untreated control (n = 10) and radiotherapy (n = 10) groups. Animals were scanned using MRI at 2 months, 4 months, and 6 months following radiotherapy (or sham radiotherapy). Mice were subject to novel object recognition tests at baseline, 1 week, 1 month, and monthly thereafter up to 6 months post-radiotherapy. All experimental procedures were carried out in accordance with the U.K Animals (Scientific Procedures) Act 1986 and EU Directive 2010/63/EU.Mice were anaesthetised and irradiated on a Small Animal Radiation Research Platform (SARRP) (XSTRAHL, US) using cone beam CT image-guidance. A single dose of 20Gy was delivered to the right hemisphere at 220kV and 13mA for approx. 7 minutes using a 5x5mm square collimator.
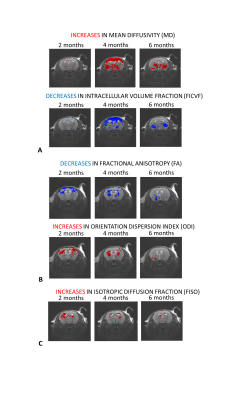
MRI was performed on an Agilant 7T 16cm bore magnet interfaced to a Bruker Avance III console. To monitor geometrical brain changes, T2-weighted TurboRARE images were acquired with TR/TE = 3592/35 ms, NEX = 10, Echo spacing = 11.6 ms, RARE factor = 8, matrix size = 256 x 256, 20 slices, slice thickness 0.375 mm, voxel size = 0.078 x 0.078 mm. To monitor microstructural changes to brain tissue, high angular resolution PGSE EPI data were acquired with TR/TE = 2000/31.5 ms, 4 EPI segments, voxel size of 0.21x0.21x0.75 mm3, 10 axial slices, 20 gradient directions at b = 300 s/mm2, 40 gradient directions at b = 700 s/mm2, 60 gradient directions at b = 2000 s/mm2 and for each shell 5 b = 0 s/mm2 images. FSL’s eddy_openmp was applied to remove subject motion and eddy current-induced distortion. FSLs DTI-FIT command was used to estimate mean diffusivity (MD) and fractional anisotropy (FA) maps. The AMICO toolbox was used to fit the NODDI model4 to derive maps of orientation dispersion index (ODI), intracellular volume fraction (FICVF), and isotropic diffusion fraction (FISO).
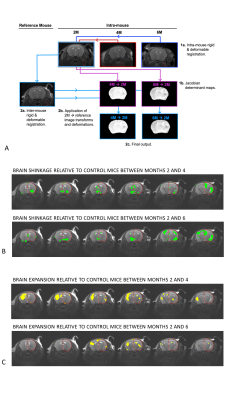
Geometrical changes of brain tissue between timepoints were determined by deformable registration5 as shown in Figure 1A. To facilitate group-level analyses, deformation fields were normalised to the standard space of a randomly selected reference mouse. The Jacobian determinant (JD) was computed from the transformed deformation fields, providing a measure of brain shrinkage (JD < 1) or expansion (JD >1) in standard space. DTI and NODDI maps were similarly transformed into standard space. All registration was done in NiftyReg (version 1.3.9). Voxelwise group-level analyses were performed in SPM using p-value threshold of 0.05 without FWE correction, and cluster threshold of 20 voxels.
Results
Radiotherapy led to brain shrinkage in the cortex, hippocampus and deep gray matter (Figure 1B). Cortical atrophy was predominantly localised to the irradiated side, and was more prevalent at 6 months compared to 4 months. Unexpectedly, brain expansion occurred in the striatum and cortex of non-irradiated cortex and was most evident at 4 months post RT.All DTI and NODDI parameters were affected by radiotherapy, but changes were transitory (Figure 2A-C). Profound MD and FICVF effects were detected at 4 months in the cortex and deep gray matter (increased MD, decreased FICVF) (Figure 2A). Decreases in FA (and increases in ODI) localised to the cortex and corpus callosum (Figure 2B) and increases in FISO localised to ventricular regions were observed, both present 2 months after radiotherapy (Figure 2C).
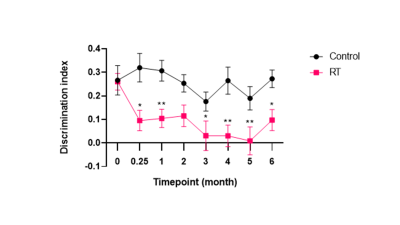
Irradiated mice suffered from deficit in NOR relative to control mice from 1 week after radiotherapy, which persisted for 6 months post radiotherapy (Figure 3; p < 0.0001). Control mice also exhibited a small reduction in NOR with time, presumably due to ageing.
Discussion
We show that hemi-brain radiotherapy leads to brain shrinkage in the cortex and hippocampal regions of the irradiated hemisphere, and expansion of cortical regions in the non-irradiated hemisphere, while microstructural changes are unilateral and appear to affect both gray (increased MD and decreased FICVF) and white matter (decreased FA, increased ODI). Effects on microstructural parameters were transitory, and had varying timescales. We hypothesize these effects may result from altered immune cell populations associated with the neuro-inflammatory response to radiation. The study is limited by small sample size and lack of baseline MRI. We plan to repeat the study with baseline imaging. We also plan to validate the microstructural changes observed with histological analyses, and use the imaging pipeline to assess the effects of novel drug-radiotherapy combinations aimed at ameliorating neurotoxicity.Acknowledgements
The study was funded by Cancer Research UK RadNet Manchester [C1994/A28701].References
[1] Khasraw M, Lassman AB. Late neurocognitive decline after radiotherapy for low-grade glioma. Nature Reviews Neurology. 2009 Dec;5(12):646-7.
[2] Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. Journal of clinical oncology. 2006 Mar 10;24(8):1305-9.
[3] Haldbo-Classen L, Amidi A, Wu LM, Lukacova S, Oettingen GV, Gottrup H, Zachariae R, Høyer M. Long-term cognitive dysfunction after radiation therapy for primary brain tumors. Acta Oncologica. 2019 May 4;58(5):745-52.
[4] Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012 Jul 16;61(4):1000-16.
[5] Modat M, Ridgway GR, Taylor ZA, Lehmann M, Barnes J, Hawkes DJ, Fox NC, Ourselin S. Fast free-form deformation using graphics processing units. Computer methods and programs in biomedicine. 2010 Jun 1;98(3):278-84.
Figures