0579
Investigating application of multi-echo, multi-delay arterial spin labeling (ASL) to study endothelial exchange of water
Swati Rane Levendovszky1, Lena Vaclavu2, Jaqueline Flores1, Elaine Peskind3, Jeffrey Iliff3, and Matthias J.P. van Osch2
1Radiology, University of Washington School of Medicine, Seattle, WA, United States, 2C.J. Gorter MRI Center, Radiology, Leiden University Medical Center, Leiden, Netherlands, 3Mental Illness Research, Education and Clinical Center, VA Puget Sound, Seattle, WA, United States
1Radiology, University of Washington School of Medicine, Seattle, WA, United States, 2C.J. Gorter MRI Center, Radiology, Leiden University Medical Center, Leiden, Netherlands, 3Mental Illness Research, Education and Clinical Center, VA Puget Sound, Seattle, WA, United States
Synopsis
Keywords: Neurodegeneration, Perfusion, sleep, aging
We applied multi-echo Hadamard encoded ASL imaging to study blood flow (CBF) and trans-endothelial exchange of water (Tex) as markers of vascular function to study two common risk factors of dementia; aging and sleep deprivation. We found that older adults had significantly lower CBF, and shorter Tex compared to young adults. In the same young adults, we compared CBF and Tex after a normal night of sleep and after sleep deprivation. Both CBF and Tex were insignificantly lower when measured after sleep deprivation compared to measurements after normal sleep.PURPOSE
The exchange of solutes as well as water transport across the endothelium are important components for maintaining vascular function and brain health1. This is especially true in aging where vascular dysfunction increases risk of cognitive impairment and dementia. In this study, we explore application of a multi-echo Hadamard-encoded multi-delay arterial spin labeling (ASL) scheme to measure, cerebral blood flow (CBF), and endothelial exchange time (T ) under two conditions: aging and sleep deprivation2,3. We, therefore, tested the hypothesis that older adults have lower CBF (reduced perfusion), and shorter Tex (leakier vessels resulting in faster exchange over the endothelium) compared to young adults. Furthermore, poor sleep quality and prolonged sleep restriction adversely affect cognitive function, and it is yet unclear whether acute sleep deprivation also affects cognitive function and whether such a change would be mediated by change in vascular function. Hence, we compared CBF, and Tex in the younger adults following a night of normal sleep and a night of sleep deprivation. Our second hypothesis is that after only one night of sleep deprivation, even young adults will have lower CBF and shorter Tex.METHODS
Experiment: Per institutional review board guidelines, ten older adults (66±3 years old, 4F) were scanned for this study. In addition, eleven young adults (22±3 years old, 5F) underwent a night of full sleep and a night of sleep deprivation. Of these, eight individuals completed both the full sleep and sleep deprivation scans. All individuals completed two cognitive tests in the morning prior to their MRI scan: General Practitioner’s assessment of cognition (GPCOG) and Memory Impairment Screen (MIS). The MRI protocol on a 3T Philips Ingenia Elition X scanner with a 32-channel head-coil: T1: we acquired a standard 1×1×1 mm3 anatomical protocol with TR/TE = 9.2/3.5. ASL: 4-block Hadamard encoded ASL with T2-prep pulses allowed for a multi-echo (TE = 0, 40, 80, 120 ms), multi-delay ASL acquisition with resolution = 3.75×3.75×5 mm3, slices = 18, label duration = 3.4 s, three effective post labeling delay = 0.65, 1.21, and 2.08 s, background suppression. A multi-echo acquisition for M0 images with identical resolution and TEs, but no labeling or background suppression was also acquired. Analysis: After rearranging data, the multi-delay ASL images were used to compute CBF and arterial arrival times using FSL, and the multi-echo ASL images were used to calculate Tex based on Ohene et al4.RESULTS
In the 10 older adults, GPCOG and MIS scores were 9±1 and 8±0. The eight younger adults showed a marginally significant difference on the GPCOG measures between full sleep (9±0) and sleep deprivation (8±1, p=0.08). No difference was observed on MIS (8±0) with changes in sleep. The multi-echo acquisition of the M0 images and the perfusion-weighted images at each of the three delay times are shown in Figure 1. CBF and Tex maps for a representative old participant and a young participant with normal sleep and sleep deprivation are shown in Figure 2. Cortical CBF (including at least 75% gray matter) in older adults, young adults with normal sleep, and the same young adults after sleep deprivation was 37±3, 41±2, 41±4, ml/100g/min respectively. Similarly, Tex values were 474±74, 609±49, 602±75 ms in the three groups respectively. The older participants had a significantly (p<0.001) lower CBF and shorter Tex compared to the young adults after full sleep. The young adults with sleep deprivation showed similar CBF and Tex compared to their values after a proper night of sleep. No significant association between Tex and cognition was observed in older adults or young adults. No significant association of CBF with cognition was detected with aging or after sleep modulation. However, percent difference in Tex values ((full sleep - sleep deprivation)/full sleep)) in the young adults was associated with corresponding percent difference in cognition (r = 0.70, p = 0.02, Figure 3).DISCUSSION
CBF and Tex were significantly reduced in older adults. Differences in CBF and Tex were not significant different after a night of sleep, but the observed change of Tex was related to difference on our cognitive tests. This might point towards poorer aquaporin-4 functioning after sleep deprivation affecting cognitive abilities, although this interpretation should be considered to be highly speculative. Furthermore, cognitive tests, sensitive to sleep-related cognitive deficits should be used in the future.CONCLUSION
Similar to many prior studies, here we confirm that CBF reduced with aging. In addition, we show that Tex is reduced i.e., water moves across the endothelium much faster with aging. These results are similar to those observed in studies of mice5. Contrary to our expectations, decreases in CBF and Tex were not observed after sleep deprivation. It is likely in acute settings of sleep deprivation, changes in vascular function are minimal and in line with observed minimal changes in cognition. Other reasons could be that our sample size was too small or that our approach is not sensitive enough to detect subtle changes such as those observed with only one night of sleep deprivation.Acknowledgements
No acknowledgement found.References
1. Iliff, Jeffrey J et al. Science translational medicine vol. 4,147 (2012):147ra111
2. Xie, Lulu et al. Science vol. 342,6156 (2013): 373-7.
3. McSorley, V Eloesa et al. American journal of epidemiology vol. 188,6 (2019): 1066-1075
4. Ohene, Yolanda, et al. Neuroimage 188 (2019): 515-523.
5. Ohene, Yolanda, et al. Magnetic resonance in medicine 85.1 (2021): 326-333.
Figures
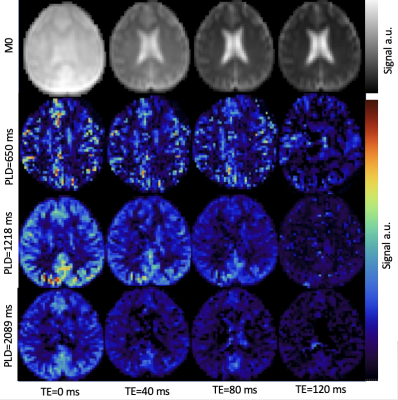
Figure 1. Our multi-echo, multi-delay ASL protocol showing M0 images in the first row, followed by the perfusion-weighted difference images at each post-labeling delay in the subsequent rows. Columns indicate acquisitions at the four different echo times.
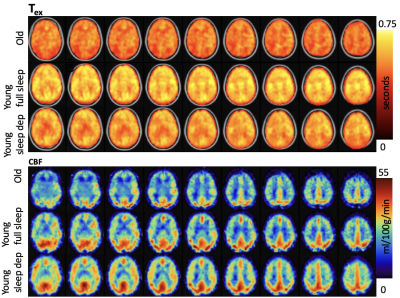
Figure 2. Maps of Tex and CBF in a representative old participant, and a young participant after a night of full sleep and the same participant after a night of sleep deprivation. Maps are overlaid on a standard 2mm, MNI template.
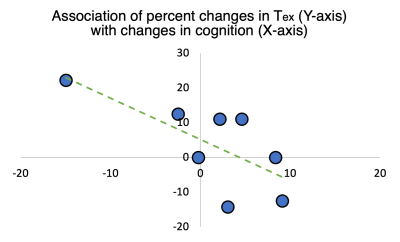
Figure 3. Difference in Tex is related to difference in cognition (r = 0.70, p =0.02), where greater Tex after sleep deprivation compared to normal sleep was associated with poorer cognition
DOI: https://doi.org/10.58530/2023/0579