0576
A longitudinal study of cerebral metabolite alteration in the developmental monkey brain with Huntington's disease1Emory National Primate Research Center, Emory University, Atlanta, GA, United States
Synopsis
Keywords: Neurodegeneration, Spectroscopy, Huntington's disease
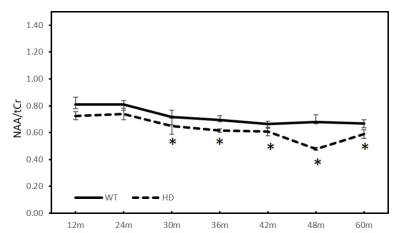
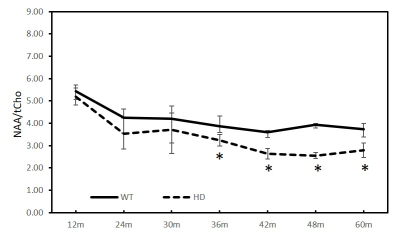
Previous studies have demonstrated in vivo MRS could be an effective approach to assess the metabolite changes in Huntington Disease (HD) patients and models. However, its sensitivity to detect the metabolite abnormality is still in dispute. The metabolic changes in the striatum of transgenic monkeys of HD were investigated with MRS from 12 to 60 months of age here. A progressive and significant reduction of NAA/tCr and NAA/tCho in striatum was observed at 30 and 36 months and old, respectively, suggesting the sensitivity of the in vivo MRS to assess the neurochemical alteration in the evolution of the disease.Introduction
Huntington’s disease (HD) is an inherited autosomal dominant neurodegenerative disorder caused by the abnormal expansion of polyglutamine (CAG) trinucleotide sequence and characterized by abnormal movements, cognition and psychiatric symptoms. Because of the high similarity in neuroanatomy, physiology, and genetics between the non-human primate (NHP) and the human, a transgenic monkey model of HD can provide a great opportunity for studying the neural substrate of the disease compared to rodents [1, 2]. Anatomical, neurochemical, and microstructural abnormities have been seen in the brains of HD patients and models in previous studies [3]. In the present, a HD monkey model was employed to examine the longitudinal changes of cerebral metabolites in the developing brain during the HD evolution.Method
Four transgenic HD rhesus monkeys and four age-matched wild-type (WT) control monkeys were used in this study [2]. In vivo MRS experiments were conducted at the age of the 12, 24, 30, 36, 42, 48 and 60 months on a 3T scanner using a customer-built single-loop surface coil (ID=5cm). During MRI scanning, animals were anesthetized using 1-1.5% isoflurane and immobilized with the home-made head holder. The physiological parameters such as End-tidal CO2, O2 saturation, blood pressure, heart rate, respiration rate, and body temperature were monitored continuously. The single voxel MRS was acquired using PRESS sequence (TR/TE =1500/30ms, FA=70°) with a 5×5×5 mm3 voxel placed in right striatum (Fig.1a). Concentrations of metabolites, including N-acetylasparlate (NAA), creatine and phosphocreatine (tCr, Cr+PCr), total choline (tCho, phosphocholine + glycophosphocholine) were derived from the spectra using the LC Model software with the unsuppressed water peak as reference. A two-way ANOVA with group as the between-subject factor and age as the within subject factor followed by post hoc analysis with p < 0.05 as the significant threshold, was performed to determine the NAA/tCr and NAA/tCho differences between groups at different ages by using SPSS.Results
As shown in Figure 2 and 3, the ratios of NAA/tCr and NAA/tCho of striatum in the HD animals showed greater decrease than those in the WT animals. Significant changes were only observed in NAA (HD vs WT, p<0.01) at 36 months, tCho (HD vs WT, p<0.05) at 42 months, and tCr (HD vs WT, p <0.05) at 12 months. However, progressive reduction of NAA/tCr and NAA/tCho was seen as early as at 12 months and reach significant reduction at 30 (Fig.2) and 36 months (Fig.3) and older, respectively.Discussion and conclusion
The striatum is a part of the thalamocortical system and its degeneration may be associated with impairment in motor and cognitive functions as well as psychiatric disturbances seen in HD models [4]. Abnormal striatal metabolic changes have been demonstrated in previous studies of HD patients [5] and animal models [2, 6]. Reduction of NAA, NAA/tCr and tCr, increased tCho in striatum of HD patients were reported [5]. However, there is the absence of conclusive finding and lack of clear longitudinal change during the HD evolution, and the role of MRS as a biomarker of HD to assess the progressive changes of striatum during the evolution of the disease is still in dispute [5]. As demonstrated in the present 5-year longitudinal study of a monkey model, NAA/tCr and NAA/tCho in striatum demonstrated progressive reductions starting as early as at 12 months; significant reduction was obtained at 30 and 36 months and older, respectively (Fig.2, and 3), suggesting the neurochemical alteration in the developmental brain during HD evolution. The significant difference of absolute quantification for NAA, tCho and tCr between WT and HD animals were hardly observed across the reported time points most likely due to the small sample size (data not shown). The present study suggested the ratios of NAA/tCr and NAA/tCho may be more sensitive to detect the progressive changes of cerebral metabolites during the HD evolution, indicating that in vivo MRS could be a robust and sensitive approach to detect early HD progress [7]. Further investigation and more associated data are needed to confirm the robustness of NAA/tCr and NAA/tCho to predict and monitor the progression of HD for translational studies and drug development.Acknowledgements
Acknowledgement: This project was funded by awarded by the ORIP/NIH (OD010930) and the office of Research Infrastructure Programs/OD P51OD011132.References
1. Meng, Y.G., et al., Developmental Whole Brain White Matter Alterations in Transgenic Huntington's Disease Monkey. Scientific Reports, 2017. 7.
2. Chan, A.W., et al., Progressive cognitive deficit, motor impairment and striatal pathology in a transgenic Huntington disease monkey model from infancy to adulthood. PLoS One, 2015. 10(5): p. e0122335.
3. Mochel, F., J.M. Dubinsky, and P.G. Henry, Magnetic Resonance Spectroscopy in Huntington's Disease. Magnetic Resonance Spectroscopy of Degenerative Brain Diseases, 2016: p. 103-120.
4. Snyder, B.R. and A.W.S. Chan, Progress in developing transgenic monkey model for Huntington's disease. Journal of Neural Transmission, 2018. 125(3): p. 401-417.
5. Arridge, E.B.J., Rachael I. Scahill, View ORCID ProfileLauren M. Byrne, Rosanna Tortelli, Amanda Heslegrave, Henrick Zetterberg, View ORCID ProfileEdward J. Wild, Longitudinal Evaluation of Magnetic Resonance Spectroscopy Metabolites as Biomarkers in Huntington’s Disease. medRxiv, 2021. https://doi.org/10.1101/2021.10.26.21265516.
6. Peng, Q., et al., Characterization of Behavioral, Neuropathological, Brain Metabolic and Key Molecular Changes in zQ175 Knock-In Mouse Model of Huntington's Disease. Plos One, 2016. 11(2).
7. Sturrock, A., et al., A longitudinal study of magnetic resonance spectroscopy Huntington's disease biomarkers. Mov Disord, 2015. 30(3): p. 393-401.
Figures
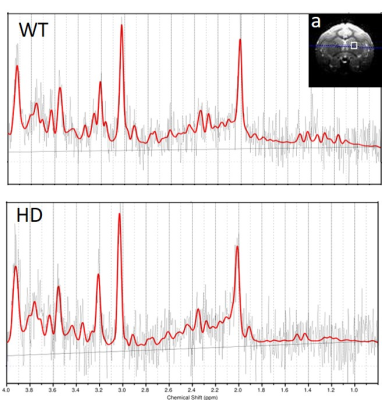
Figure.1 The location of striatum (a) and representative spectra from a WT (wild type) and a HD (Huntington's disease) monkeys.