0575
Diffusion MRI reveals rescue of structural changes in a mouse model of Huntington’s disease by mutant Huntingtin lowering1Bio-Imaging Lab, Department of Biomedical Sciences, University of Antwerp, Antwerp, Belgium, 2µNEURO Research Centre of Excellence, University of Antwerp, Antwerp, Belgium, 3CHDI Management/CHDI Foundation, Princeton, NJ, United States
Synopsis
Keywords: Neurodegeneration, Diffusion/other diffusion imaging techniques, Huntington's Disease
Huntington’s disease (HD) is a neurodegenerative disorder for which no cure is available. There is an urgent need to find early biomarkers which are sensitive enough to determine and follow up the efficacy of novel therapies. In this study, we present the outcome of a treatment study in the LacQ140 HD mouse model using diffusion tensor/kurtosis imaging (DTI/DKI) and fixel-based analysis (FBA). We observed that early, moderate lowering of mutant Huntingtin (mHtt) rescues structural alterations measured in this mouse model with diffusion MRI in the olfactory bulb and striatum, which are prominent regions affected in HD.
Introduction
Huntington’s disease (HD) is an autosomal neurodegenerative disorder characterized by neuropsychiatric, cognitive, and motor symptoms. To date there is no cure available for this fatal disease1. To facilitate the development of novel therapies, there is an urgent need to find early biomarkers which are sensitive enough to determine and follow up the efficacy of a new treatment. In a previous study, Vidas-Guscic et al. (2022) used diffusion kurtosis imaging (DKI) and fixel-based analysis (FBA) in the zQ175DN mouse model for HD and revealed micro- and macro-structural changes in HD relevant regions such as the corpus callosum and the striatum2. As a next step, we used these optimized acquisition and processing protocols to investigate which structural changes are rescued if the mutant Huntingtin (mHtt) expression is lowered in the LacQ140 HD mouse model. This allowed us to validate these diffusion imaging biomarkers and more importantly, to assess their sensitivity to detect treatment effects.Methods
We used the heterozygous LacO/LacIR-regulatable LacQ140 HD mouse model3 in which mHtt transcription is regulated by isopropyl-ß-d-1-thiogalactopyranoside (IPTG) in a time-controlled manner. The LacQ140 group had over their life span continuous administration of IPTG, resulting in high levels of mHtt 4. In the LacQ140_2M group, IPTG exposure was withdrawn starting from 2 months of age to lower mHtt expression by 40% (Fig. 1). Our study included 11-month-old mice from the LacQ140 group (N=10 males/10 females), the LacQ140_2M group (N=10 males/9 females), and the wild-type (WT) control group (N=9 males/10 females) also under continuous IPTG administration. The diffusion-weighted MRI data were acquired under 1-2% of isoflurane on a 9.4 T Biospec MRI scanner (Bruker, Germany), with a mouse head cryo-coil (Bruker, Germany) using a multi-shell (9 b = 0 s/mm2, b = 800/1200/2800 s/mm2 with respectively 45/60/75 diffusion directions, δ = 3.5ms, Δ = 9.84ms) 4-shot spin echo diffusion-weighted EPI sequence (TR/TE = 4000/20 ms). Eighteen horizontal slices (400 µm) were acquired with an in-plane resolution of (300 x 300) µm2 and a matrix size of [80 x 92]. Images were pre-processed using Gibbs ringing, noise-, motion-, distortion and bias-field correction followed by diffusion kurtosis analysis and fixel modeling (MRtrix3.0) 5. Two-way ANOVA (genotype, gender and genotype x gender) with FDR correction (p<0.05) was used to statistically analyze diffusion metrices of HD-relevant regions-of-interest (ROI) (Fig.2).Results
The area in between the two olfactory bulbs (abOB) demonstrated significant main genotype effects in mean diffusivity (MD), axial diffusivity (AD), radial diffusivity (RD), axial kurtosis (AK), fiber bundle cross-section (FC) and fiber density cross-section (FDC). Post-hoc tests in the abOB revealed an increased diffusivity (MD, AD, RD) in both the LacQ140 and LacQ140_2M groups compared to the WT group, but the diffusivity in the LacQ140_2M was significantly lower compared to the LacQ140 group. (Fig. 3A-4). Significant reductions in FC, FDC and AK in abOB were found in the LacQ140 compared to the WT group, while the differences in the WT and the LacQ140_2M group were non-significant (Fig. 4). In the corpus callosum, FC and FDC were decreased in the LacQ140 and the LacQ140_2M groups compared to the WT group (Fig. 3B-4). The striatum demonstrated increased kurtosis values (MK and RK) in the LacQ140 group compared to the WT group while there were no significant differences between the WT and the LacQ140_2M groups (Fig. 3C-4). FDC was decreased in the striatum of the LacQ140 group compared to the WT group, but to a lesser extent in the LacQ140_2M group (Fig.4). In the motor related areas, an interaction effect was found for MK and RK between genotype and gender (Fig. 4). This diffusion MRI study also revealed significant main gender effects in multiple ROIs.Discussion
Partial lowering of mHtt from 2 months of age onwards in the LacQ140 mouse model rescues some of the structural HD pathology that can be measured with diffusion MRI at 11 months of age. Fixel and kurtosis measures are sensitive to detect genotypic differences and mHtt lowering effects in the olfactory bulb and striatum which are relevant regions for HD. Except for MK and RK in the motor related areas, none of the diffusion metrices demonstrated an interaction effect between genotype and gender in the selected ROIs. This indicates that, although we find overall gender differences, structural changes in this mouse model and the effects of lowering mHtt on them are not gendered. The selected region in the olfactory bulb was delineated based on an exploratory whole brain voxel-based analysis, but these results should be interpreted with caution. As there is no anatomically defined tract, we could hypothetically ascribe these differences to changes in CSF flow or local shape changes.Conclusion
DKI and FBA provide sensitive methods to detect the micro- and macro-structural effects of early mHtt lowering in the LacQ140 mouse model for HD. Therefore, diffusion kurtosis and fixel measures are currently considered as candidate biomarkers for future research to test and follow up the efficacy of mHtt lowering agents in HD. Histology is warranted to better understand the micro- and macro-structural effects associated with HD pathology.Acknowledgements
This work was funded by CHDI Foundation, Inc., a nonprofit biomedical research organization. The computational resources and services were provided by the HPC core facility CalcUA, the VSC, funded by the Hercules Foundation, and the Flemish Government department EWI. The Bruker Biospec 9.4T system (Bruker, Ettlingen, Germany) was upgraded to AVANCE-NEO through Hercules foundation funding (Belgium, grant agreement I007120N) under the promoter-ship of AVDL.References
1. Bates, G., Dorsey, R., Gusella, J. et al. Huntington disease. Nat Rev Dis Primers 1, 15005 (2015).
2. Vidas-Guscic, N. et al. Longitudinal Fixel-Based Analysis of diffusion MRI in the zQ175 Huntington’s disease mouse model. Proc 31st Annual meeting ISMRM (2022).
3. Marchionini, D. et al. Benefits of global mutant huntingtin lowering diminish over time in a Huntington’s disease mouse model. JCI Insight. 7(20):e161769 (2022).
4. Bertoglio, D. et al. Development of a ligand for in vivo imaging of mutant huntingtin in Huntington’s disease. Sci Transl Med 14, eabm3682 (2022).
5. Tournier, J.D. et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 202, 116137 (2019).
Figures
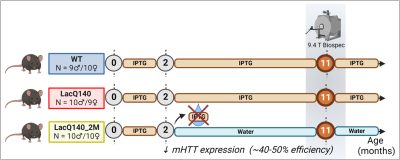
Figure 1: Sample sizes for the diffusion MRI study in the LacQ140 HD mouse model. Both the WT (9 males/10 females) and the LacQ140 (HD) group (10 males/9 females) were exposed to IPTG over their complete life span. In the LacQ140_2M group (10 males/10 females), IPTG was withdrawn at the age of 2 months, resulting in a decreased mHtt expression. All groups were scanned later at 11 months of age. Figure created with Biorender.com
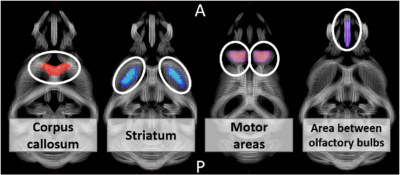
Figure 2: Four regions-of-interest visualized on a study-based tract density image. Per subject the mean voxel/fixel values from the diffusion metrices within these regions/tracts were calculated. A = anterior, P = posterior.
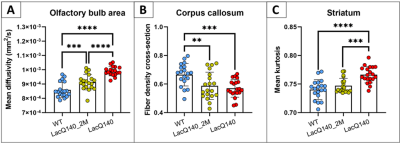
Figure 3: Post-hoc tests of the main group effects demonstrated specific group differences for the mean diffusivity in the olfactory bulb area (A), the fiber density cross-section in the corpus callosum (B), and the mean kurtosis in the striatum (C). ** p<0.01; *** p<0.001; **** p<0.0001 (FDR corrected)
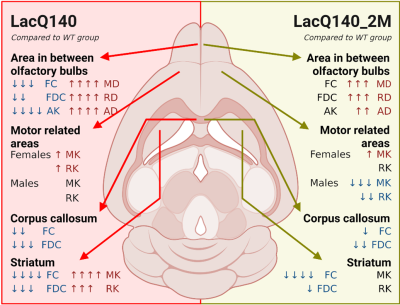
Figure 4: Summary of the results from the region-of-interest analysis. Number of arrows indicate significance level (*) compared to the WT group. * p<0.05; ** p<0.01; ***p<0.001; ****p<0.0001. ↑ increase compared to the WT group; ↓ decrease compared to the WT group. Figure created with Biorender.com